Unit of Reproductive Endocrinology, 1st Department of Obstetrics & Gynecology, Hippocration General Hospital, Aristotle University of Thessaloniki, 546 42 Thessaloniki, Greece
β-thalassemia (β-thal) is characterized by disturbances of the reproductive system. The aim of the present study was: 1) to assess the hypothalamic _ pituitary - gonadal axis in patients with β-thal in relation to their phenotype and 2) to determine prognostic features of current gonadal status. We studied 135 patients (67 males and 68 females) with β-thal through history, physical examination, spermiograms and GnRH test. These patients were divided into β-thal major (51 males and 62 females) and β-thal intermedia phenotypes (16 males and 6 females). Male patients with β-thal major were subdivided into three groups a) eugonadal (35%, Tanner's stage V, normal testicular volume, normal spermiograms, normal basal and stimulated hormone values), b) patients with hypogonadotrophic hypogonadism (HH) of late onset (24%, Tanner's stage II-V, low-normal testicular volume, abnormal spermiograms, normal basal gonadotrophin values and abnormal response to GnRH test) and c) patients with HH of early onset (41%, Tanner's stage I, small testicular volume, abnormal spermiograms, abnormal basal and stimulated hormone values). Female patients with β-thal major were subdivided into: a) eugonadal (32%, Tanner's stage V, regular menstruation, normal basal and stimulated hormone values), b) patients with hypogonadotrophic hypogonadism (HH) of late onset (34%, Tanner's stage II-V, secondary amenorrhea, subnormal basal and stimulated gonadotrophin values) and c) patients with HH of early onset (34%, Tanner's stage I, primary amenorrhea, subnormal basal and stimulated hormone values). Patients with β-thal intermedia were subdivided into eugonadal (75% of males - 33% of females) and hypogonadal (25% of males - 67% of females). Current gonadal status could not be predicted by means of transfusion or chelation parameters. In conclusion, β-thal patients could be eugonadal or develop early or late onset HH. ™-thal intermedia patients have a more favorable profile than β-thal major individuals. Current gonadal status of β-thal patients cannot be predicted by means of history, clinical or laboratory parameters.
β-thalassemia major, β-thalassemia intermedia, gonadal axis, GnRH test, spermiogram
INTRODUCTION
Homozygous beta-thalassemia (β-thal), an autosomal recessive disorder, is characterized by a reduction or absence of normal β-globin synthesis1. The genes for β-thal are prevalent in several ethnic groups distributed in a broad geographic belt from the Mediterranean basin through the Middle East and the Indian subcontinent and into southeast Asia2. β-thal is a serious genetic disorder which results in a considerable increase in both acute and chronic morbidity and mortality3. Failure of pubertal growth, delay or absence of sexual development, amenorrhea, sexual dysfunction and infertility due to hypogonadism are well-recognized disturbances of the hypothalamic " pituitary " gonadal axis in β-thal patients4. Aberrant gonadotrophin response to GnRH administered acutely and in a pulsatile fashion strongly indicates failure of gonadotroph cells, which seem to be extremely vulnerable to iron damage5.
β-thal has extremely variable clinical phenotypes. Extensive investigations carried out in the last two decades have to a great extent defined the molecular basis of the phenotypic heterogeneity of β-thal syndromes. Differentiation between the main clinical phenotypes of β-thal major and β-thal intermediate is critical for appropriate treatment.
In the past, children with β-thal rarely survived beyond adolescence6. The improved expectancy and quality of life in β-thal patients due to introduction in the late 1970s of regular optimum red blood cell transfusions and almost daily subcutaneous iron chelation therapy made the necessity for normal reproductive and sexual life more pressing7.
Although thousands of people worldwide have β-thal and therefore are at increased risk for reproductive health problems, the literature on these issues is scarce, particularly the relation between clinical phenotype and gonadal axis status.
The aim of the present study was: 1) to assess the hypothalamic - pituitary - gonadal axis in patients with β-thal according to their phenotype and 2) to relate history, clinical and laboratory parameters with current gonadal status in an attempt to define prognostic factors of current gonadal status.
SUBJECTS & METHODS
Patients. The study group included 135 patients (67 males and 68 females) with β-thal. The patients were divided into two groups: 113 patients with β-thal major (51 males and 62 females) and 22 patients with β-thal intermedia (16 males and 6 females). Inclusion criterion for the β-thal intermedia subgroup was the ability to maintain a hemoglobin level compatible with comfortable survival in the absence of regular transfusions. The 135 patients constituted the total number of referrals to the Unit of Reproductive Endocrinology from five district Thalassemia Units, throughout Northern Greece, during a decade (1991-2000). Patients' characteristics are given in table 1 .
Study characteristics. Cross-sectional, clinical - laboratory study.
Methods. A uniform questionnaire was used in order to collect history information. Sexual maturation was assessed using the criteria proposed by Tanner8. Testicular volume was evaluated by the Prader orchidometer. All patients were clinically evaluated by a single experienced physician (J.P.).
The Unit of Reproductive Endocrinology where the study was conducted is the referral infertility center for the whole of Northern Greece. Although every possible effort has been made for all β-thal patients to be referred to the Unit for gonadal axis evaluation regardless of their gonadal status, there was no way of confirming that this had actually happened. Thus, our population can be described most accurately as a selective cohort of Greek patients with β-thal.
Ferritin was measured in a random blood sample at the time of referral to our Unit. Therefore it is most probable that the ferritin values are not representative of the mean ferritin levels of the patient throughout the previous years.
A 100 µg gonadotrophin-releasing hormone (GnRH) test (Relefact, Hoechst) was performed in order to evaluate pituitary reserve for gonadotrophins. Samples were obtained at 0, 30 and 60 minutes. Gonadotrophins were measured by ELISA (IMX Abbott Labs), reference range of basal values FSH: 1-15 mIU/ml (males), 2-15 mIU/ml (females, follicular phase), expected peak FSH: 10-35 mIU/ml (males and females), basal values LH: 3-25 mIU/ml (males), 2-20 mIU/ml (females, follicular phase), expected peak LH: 10-35 mIU/ml (males and females). Basal levels for free testosterone measured by ELISA: 16-39 ng/dl (males, 20 to 40 years old), estradiol measured by ELISA: 30-300 pg/ml (females, follicular phase) and sex-hormone binding globulin (SHBG) measured by ELISA: 20-130 nmol/l (females).
Sperm samples were collected by masturbation after 3-4 days of abstinence. All samples were evaluated according to World Health Organization (WHO) criteria9. A minimum of two spermiograms was obtained at 3- month intervals.
Statistics. Data are expressed as mean ± standard error of the mean (SEM). Chi-square test was used in order to search for differences regarding the means in nominal parameters whereas Mann-Whitney U (for comparisons between 2 groups) and Kruskal-Wallis (for comparisons in more than 2 groups) were used for interval data. A stepwise discriminant analysis was performed using the Wilks' Lambda statistic in order to weigh the addition or removal of variables from the procedure. Statistical analysis was performed using the SPSS 10 statistical package (SPSS Inc, Chicago, Ill.).
RESULTS
Clinical features and laboratory profiles. On the basis of clinical and laboratory parameters, male β-thal major patients were divided into three subgroups (Table 2a ). Group A (eugonadal): eighteen patients (35%) who had normal sexual development (Tanner's stage V), normal testicular volume, normal spermiograms and normal basal and stimulated gonadothophin values. Group B: (hypogonadotrophic hypogonadism of late onset): twelve patients (24%) who had a variable degree of sexual development (Tanner's stage II-V), low-normal testicular volume, abnormal spermiograms, normal basal gonadotrophin values and abnormal response to GnRH test (Figure 1). These patients had either arrested puberty (Tanner's stage II-IV) or full development of secondary sexual characteristics (Tanner's stage V) and subsequently developed HH. Group C: (HH of early onset): twenty-one patients (41%) who were prepubertal (Tanner's stage I) with small testes, abnormal spermiograms and low basal and stimulated hormone values. On the basis of the same clinical and laboratory parameters, male β-thal intermedia patients were subdivided into eugonadal (n=12 - 75%) and hypogonadal (n=4 - 25%) (Table 2b) subjects.
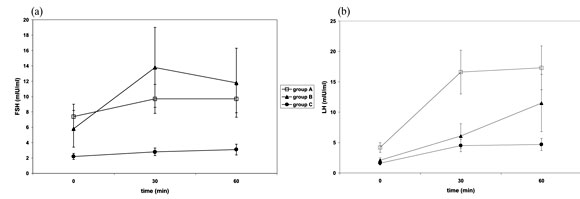
Figure 1. GnRH test results in male patients with β-thal major - (a) FSH and (b) LH values. Group A: eugonadal, Group B: hypogonadotrophic hypogonadism - late onset, Group C: hypogonadotrophic hypogonadism - early onset.

Similarly, women with β-thal major were divided into three subgroups (Table 3a ). Group A: (eugonadal): twenty patients (32%) who had normal sexual development (Tanner's stage V), regular menstruation and normal basal and stimulated gonadotrophin values. Group B (HH of late onset): twenty-one patients (34%) who had a broad spectrum of sexual development (Tanner's stage II-V) with secondary amenorrhea and subnormal basal and stimulated gonadotrophin values (Figure 2). These patients had either arrested puberty (Tanner's stage II-IV) or full development of secondary sexual characteristics (Tanner's stage V) followed by the development of HH at a later age. Group C (HH of early onset): t wenty-one patients (34%) who were prepubertal (Tanner's stage I) and had subnormal basal and stimulated gonadotrophin values. On the basis of the same clinical and laboratory parameters, female β-thal intermedia patients were subdivided into eugonadal (n=2 - 33%) and hypogonadal (n=4 - 67%) (Table 3b).

Figure 2. GnRH test results in female patients with β-thal major - (a) FSH and (b) LH values. Group A: eugonadal, Group B: hypogonadotrophic hypogonadism - late onset, Group C:hypogonadotrophic hypogonadism - early onset.

Males with β-thal intermedia had higher age at referral, starting age of transfusions, starting age of chelation therapy and lower risk of hepatitis B and hepatitis C antibody positivity as compared to males with β-thal major (Mann-Whitney U, p<0.05, Table 1). In addition, males with β-thal intermedia as compared to β-thal major males had greater mean testicular volumes, higher basal and stimulated LH levels, higher basal free T levels and reduced risk of being hypogonadal (Tables 2a and 2b). There was no statistically significant difference in sperm parameters.
Similarly, females with β-thal intermedia were older at referral, at first transfusion and at start of chelation therapy as compared to females with β-thal major (Mann-Whitney U, p<0.05, table 1). They were less frequently transfused and they presented lower risk for hepatitis B and hepatitis C as predicted by antibody positivity. On the other hand, no statistically significant differences in clinical or laboratory parameters were demonstrated in females with β-thal intermedia as compared to β-thal major females (tables 3a and 3b).
Prognostic factors for gonadal status. By means of a discriminant analysis, there was no parameter from patients' history that could predict current gonadal status of β-thal patients. Moreover, there was no statistically significant correlation between gonadal status and starting age of transfusion therapy, transfusion pattern, serum hemoglobin, starting age of chelation therapy, chelation therapy pattern or serum ferritin. Likewise, there was no significant correlation between current gonadal status and time interval between starting age of transfusion and starting age of chelation therapy.
On the other hand, there was statistically significant correlation between testicular volume and semen para-meters (sperm count, motility at 1 hour and morphology - Kendall's tau-b, p<0.05), as well as testicular volume and hormone values (FSH / LH at 0, 30 and 60 minutes and free testosterone at 0 minutes - Kendall's tau-b, p<0.01).
Other results. Four of the β-thal male patients were married (one β-thal major eugonadal, one β-thal intermedia eugonadal and two β-thal major HH-late onset) but none had children. On the other hand, six of the β-thal female patients were married (four eugonadal and two HH-late onset) and four of them have given birth to five children (two β-thal major eugonadal patients and one β-thal intermedia eugonadal patient with one child each and one β-thal intermedia eugonadal patient with two children).
DISCUSSION
Approximately 10% of patients with homozygous β-thal exhibit a phenotype characterized by intermediate hematologic severity due to a less severe defect in β-globin synthesis than in typical β-thal major. The patients with β-thal intermedia have a milder clinical phenotype as they co-inherit a form of ˜-thalassemia or they carry β-thal chromogenes with a higher than usual potential for high levels of š-globin expression. Some of these patients can survive well into adult life, usually with normal onset and progression of puberty. As patients with β-thal intermedia pose therapeutic dilemmas and constitute a group of special interest among physicians involved with thalassemia, we have studied them as a separate subgroup.
In our study, 65% of male and 68% of female patients with β-thal major developed HH in contrast to 25% of males and 67% of females with β-thal intermedia. Tolis et al. have reported a higher incidence of hypogonadism in Greek subjects. In their series of 410 female β-thal patients, gonadal dysfunction, manifested as menstrual irregularities and hypoestrogenemia was demonstrated in 95% of cases. However, only 74% of patients developed primary or secondary amenorrhea10. In a previous study of our group, 40% of male patients with β-thal were found to be hypogonadal11.
A direct comparison with non-Greek studies is not straightforward due to the great variety of β-thal genotypes and phenotypes in different parts of the world. In any case, Kwan et al. have reported similar results; 62% of boys and 75% of girls over the age of 12 years had HH, despite regular transfusion and chelation therapy12. Borgna-Pignatti et al. found in their β-thal group a complete lack of pubertal changes in 38% of females and 67% of males aged 12 to 18 years. Only 19% of females had experienced menarche but a third of them developed secondary amenorrhea.
The majority of researchers agree that in patients with β-thal intermedia, gonadal function is preserved and pubertal timing is not disturbed14. We have found a statistically significant lower incidence of hypogonadism in β-thal intermedia male patients as compared to β-thal major individuals. We were not able to demonstrate the same effect in female patients, possibly due to small sample size.
The damage to the hypothalamic -pituitary- gonadal axis is most likely localized at a central level. The classic knowledge is that in transfusion-dependent β-thal patients, increased iron deposition in the pituitary gland has a cytotoxic effect, leading mainly to HH due to pituitary hyporesponsiveness to GnRH15. HH does not respond to chelation therapy given late in the course of the disease16. In our study, neither male nor female patients were found to have hypergonadotrophic hypogonadism. In general, hypogonadal male patients with β-thal intermedia had higher basal and stimulated LH levels than hypogonadal males with β-thal major (tables 2a and 2b) suggesting higher pituitary reserve. On the other hand, higher LH plasma levels do not necessarily denote better pituitary gonadotroph function as ˜-subunit abnormalities have been suggested in previous studies17.
Hyperprolactinemia seems not to be implicated in the pathogenesis of HH in β-thal patients15. In all male and female hypogonadal patients in whom plasma prolactin levels were measured, they were found to be within the normal range (data not shown).
The progressive nature of the disease makes all eugonadal male β-thal patients candidates for developing premature forms of partial androgen deficiency of the aging male (PADAM). Therefore, these patients must enter a follow-up program in order for hypogonadism to be diagnosed early and treated appropriately.
The study was not designed to detect fertility rate in β-thal patients. Due to small sample size, no useful conclusions can be drawn regarding the clinical and laboratory characteristics of β-thal patients who became parents. However, the low marriage rate (5-6%) in β-thal patients cannot be attributed exclusively to hypogonadism. Previous studies have shown that β-thal patients experience substantially more emotional problems, social isolation and stigmatization than their peers, suggesting that these people have limited opportunities for normal sexual behavior and social development18,19.
In our study, a discriminant analysis failed to determine parameters that could predict current gonadal status. A literature review revealed that other researchers experienced similar difficulties. Weintrob et al. suggested that beginning chelation treatment with desferrioxamine before the onset of puberty can help children with transfusion-dependent β-thal to attain normal sexual maturation20. Conversely, Soliman et al. stated that despite regular blood transfusions and desferrioxamine treatment, growth impairment and pubertal delay are commonly seen in children and adolescents with transfusion-dependent β-thal21. A multiple regression analysis of indicators of pubertal development with age, age at first transfusion, age at splenectomy, number of transfusions, serum transaminase and ferritin, and duration and intensity of chelation therapy failed to identify the factors responsible for the variation observed in sexual maturation among patients with β-thal13. To the best of our knowledge, there are no studies that identified prognostic pavameters for gonadal status in patients with β-thal.
In conclusion, we conducted a cross-sectional study in 135 Greek patients with β-thal major and β-thal intermedia. The former can be classified by means of clinical and laboratory criteria into three groups: eugonadal patients and patients with early or late onset HH, whereas the latter can be classified into eugonadal and hypogonadal. β-thal intermedia patients have a more favorable profile than β-thal major individuals regarding gonadal function. No parameter from patients' histories can predict current gonadal status. Nevertheless, modern state-of-the-art management has contributed towards an improvement in quality of life in patients with β-thal thus making the desire for normal reproductive and sexual life more pressing.
ACKNOWLEDGMENTS
We would like to thank all the physicians and nursing staff of 2nd Department of Internal Medicine, Aristotle University of Thessaloniki, Greece, as well as the staff of the Thalassemia Units of AHEPA, Hippocration, Karditsa and Volos General Hospitals for their invaluable help in data collection and clinical management of the patients.
REFERENCES
1. Weatherall DJ, 1998 Pathophysiology of thalassaemia. Baillieres Clin Haematol 11: 127-146.
2. Pearson H, Cohen A, Giardina P, Kazazian H, 1996 The changing profile of homozygous β-thalassemia: demography, ethnicity, and age distibution of current North American patients and changes in two decades. Pediatrics 97: 352-356.
3. Olivieri NF, 1999 The beta-thalassemias. N Engl J Med 341: 99-109.
4. Thein SL, 1998 Beta-thalassaemia. Baillieres Clin Haematol 11: 91-126.
5. Clarke GM, Higgins TN, 2000 Laboratory investigation of hemoglobinopathies and thalassemias: review and update. Clin Chem 46: 1284-1290.
6. Politis C, Di Palma A, Fisfis M, Giasanti A, Richardson A, Vullo C, 1990 Social integration of the older thalassemic patients. Arch Dis Child 65:984-986.
7. Rodgers GP, 1998 Pharmacological therapy. Baillieres Clin Haematol 11: 239-255.
8. Tanner J, Whitehouse R, Takaishi M, 1966 Standards from birth to maturity for height, weight, height velocity and weight velocity in British children. Arch Dis Child 41: 454-458.
9. World Health Organization,1992 WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press.
10. Tolis G, Karagiorga M, 1999 Aberrant gonadotrope behavior in 302 amenorrheic patients with ™-thalassemia major. The 7th International Conference on Thalassemia and the Hemoglobinopathies. Bangkok, Thailand.
11. Papadimas J, Mandala E, Pados G, Kokkas B, Georgiadis G, Tarlatzis B, et al, 1996 Pituitary-testicular axis in men with beta-thalassaemia major. Hum Reprod 11: 1900-1904.
12. Kwan EY, Lee AC, Li AM, Tam SC, Chan CF, Lau YL, et al, 1995 A cross-sectional study of growth, puberty and endocrine function in patients with thalassaemia major in Hong Kong. J Paediatr Child Health 31: 83-87.
13. Borgna-Pignatti C, De Stefano P, Zonta L, Vullo C, De Sanctis V, Melevendi C, et al, 1985 Growth and sexual maturation in thalassemia major. J Pediatr 106: 150-155.
14. Tolis GJ, Vlachopapadopoulou E, Karydis I, 1996 Reproductive health in patients with beta-thalassemia. Curr Opin Pediatr 8: 406-410.
15. Allegra A, Capra M, Cuccia L, Pulejo ML, Raineri L, Corselli F, et al, 1990 Hypogonadism in beta-thalassemic adolescents: a characteristic pituitary-gonadal impairment. The ineffectiveness of long-term iron chelation therapy. Gynecol Endocrinol 4: 181-191.
16. Wang C, Tso SC, Todd D, 1989 Hypogonadotropic hypogonadism in severe beta-thalassemia: effect of chelation and pulsatile gonadotropin-releasing hormone the-rapy. J Clin Endocrinol Metab 68: 511-516.
17. Tato L, Lahlou N, Zamboni G, De Sanctis V, De Luca F, Arrigo T, et al, 1993 Impaired response of free alpha-subunits after luteinizing hormone-releasing hormone and thyrotropin-releasing hormone stimulations in beta-thalassemia major. Horm Res 39: 213-217.
18. Ratip S, Skuse D, Porter J, Wonke B, Yardumian A, Modell B, 1995 Psychosocial and clinical burden of thalassemia intermedia and its implications for prenatal diagnosis. Arch Dis Child 72:
19. Aydin B, Yarpak I, Akarsu D, Okten N, Ulgen M, 1997 Psychosocial aspects and psychiatric disorders in children with thalassemia major. Acta Pediatr Jpn 39: 354-357.
20. Bronspiegel-Weintrob N, Olivieri NF, Tyler B, Andrews DF, Freedman MH, Holland FJ, 1990 Effect of age at the start of iron chelation therapy on gonadal function in beta-thalassemia major. N Engl J Med 323: 713-719.
21. Soliman AT, el Zalabany M, Amer M, Ansari BM, 1999 Growth and pubertal development in transfusion-dependent children and adolescents with thalassaemia major and sickle cell disease: a comparative study. J Trop Pediatr 45: 23-30.
Address correspondence and requests for reprints to:
Dr. Dimitrios G. Goulis, Kizikou 20, GR-551 33 Kalamaria
Greece, tel: + 3-0310480.636, e-mail: dgg30@otenet.gr
Received 29-10-02, Revised 2-04-02, Accepted 15-06-02