Department of Pathology G. Gennimatas Athens General Hospital, Athens, Greece
OBJECTIVE: The term “null cell” adenoma was first proposed in 1980 to designate pituitary adenomas lacking clinical, biochemical and morphological markers to disclose their cell origin. DESIGN: The aim of this study was to investigate the presence of α- and β-gonadotropin subunits in clinically nonfunctioning pituitary tumors, which were initially immunonegative and thus diagnosed as null cell adenomas. For this reason, we reapplied immunohistochemistry using a more sensitive method comprising a tyramide signal amplification technique, combined with a polymer antibody immunohistochemical detection system. RESULTS: With this approach, all these previously negative tumors became positive for α- and β-gonadotropin hormone subunits. CONCLUSIONS: Our results prove that so-called “null cell” adenomas produce α-SU or/and β-FSH or β-LH and therefore are gonadotrph adenomas in origin.
Adenoma, Glycoprotein hormones, Gonadotroph adenoma, Immunohistochemistry, Null-cell adenoma, Pituitary
INTRODUCTION
The tern “null cell” adenoma was first proposed by Kovacs et al in 1980 to designate pituitary adenomas lacking clinical, biochemical and morphologic markers, which would permit disclosure of their cellular origin. This original study was based on a series of surgically removed pituitary adenomas, which were unassociated clinically or biochemically with hormone secretion in excess. By immunohistochemistry, a substantial majority of these chromophobic tumors contained no adenohypophysial hormones. Electron microscopy also failed to reveal their morphogenesis.1 However, in our material only a small subset of nonfunctioning adenomas was immunonegative for all pituitary hormones.
The aim of our study was to reexamine nonfunctioning pituitary adenomas, diagnosed as null cell, which were initially immunonegative for α- and β-subunits of gonadotropin hormones. To investigate gonadotropin hormone production by null cell adenomas, we repeated the immunohistochemistry using advanced techniques, including the sensitive catalyzed tyramide signal amplification (TSA) technique, combined with a polymer antibody containing detection system.
MATERIAL AND METHODS
Among 1492 surgically removed pituitary adenomas, collected at the National Pituitary Tumor Reference Center, 51 (3.4%) were chromophobic, PAS-negative and negative for all pituitary hormones, null cell adenomas. In addition, all 51 adenomas were nonfunctioning, since patients harboring these tumors showed no clinical signs or symptoms attributed to GH-, PRL-, ACTH-, or TSH-hypersecretion. For this study, 10 of these adenomas were randomly selected.
The initial diagnosis was based on immunohistochemistry using the standard avidin-biotin-peroxidase (ABC) method, after enzymatic pretreatment with pronase to reveal the hidden antigenic sites. Polyclonal antisera were directed against growth hormone (GH), prolactin (PRL), adrenocortinotropin (ACTH) and β-subunit of thyrotropin hormone (β-TSH), β-follicle stimulating hormone (β-FSH), β-luteinizing hormone (β-LH) and α-subunit of glycoprotein hormone (α-SU). The working dilutions ranged from 1:200 to 1:2.000. All antisera were provided by the National Hormone and Peptide Program (NHPP, Torrance CA, USA). Details of the method were described previously.2
In the current study, immunohistochemistry was repeated using the same pretreatment with pronase and the same dilutions for all pituitary hormone antisera. The next day, sections were treated with a signal amplification system, comprising peroxidase catalyzed deposition of biotinylated TSA kit (Dako A/S, Glostrup Denmark), followed by the one-step EnVision polymer detection system (Dako A/S, Glostrup Denmark) as a secondary link to DAB chromogen.3,4 Two sets, each one of 7 sections from gonadotroph adenomas, known as positive for β-FSH β-LH and α-SU and negative for the remaining hormones (GH, PRL, β-TSH and ACTH), served as controls. They were immunostained together with the study group of the 10 null cell adenomas followed by TAS and detection system procedures. Sections from the first set immunostained with the 7 pituitary hormone antisera served as positive controls. Sections of the second set, where the primary antisera were substituted by buffer solution, served as negative controls.
For electron microscopy, samples from the adenomas were fixed in glutaraldehyde, postfixed in osmium tetroxide and embedded in Epon-Araldite. Ultrathin sections stained with uranyl acetate and lead citrate were examined on either a Philips 300 or a 410-LS electron microscope.2
RESULTS
Immunohistochemical studies
After application of the TSA technique, all 10 tumors became positive for pituitary α- and β-gonadotropin hormone subunits. The immunoreactivity was heterogeneous regarding the distribution, extent and staining intensity (Figure 1). Immunostains were diffuse in the cytoplasm, often with enhancement along cytoplasmic membranes (Figure 2). In some tumors, the attenuated cytoplasmic processes of adenoma cells were highlighted. In addition, the immunoreactivity in several cells of the positive control specimens of gonadotroph adenomas that was previously mild to moderate became more intense (Figure 3). Immunostains were negative for the remaining pituitary hormones (GH, PRL, ACTH and β-TSH) in all studied cases and positive controls. Negative controls were immunonegative for all pituitary hormones.
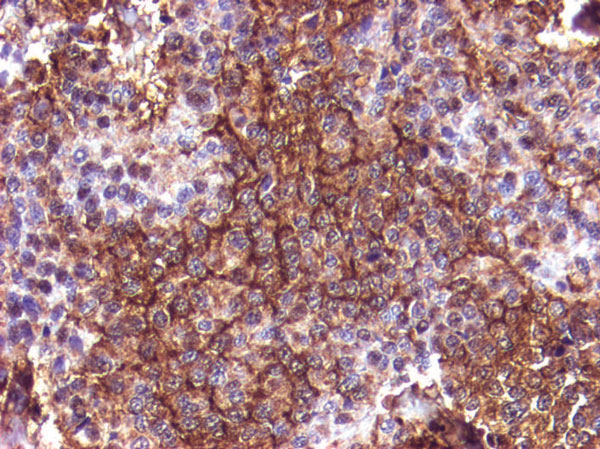
Figure 1. Adenoma strongly positive for beta-LH. Immunoreactivity depicting cytoplasmic granularity is heterogeneous. Some cells show enhancement along cytoplasmic membranes (TSA- EnVision system, original magnification 20×).

Figure 2. Adenoma strongly positive for beta-FSH. Some cells show elongated cytoplasmic processes, characteristic for glycoprotein hormone cell differentiation (TSA- EnVision system, original magnification 20×).
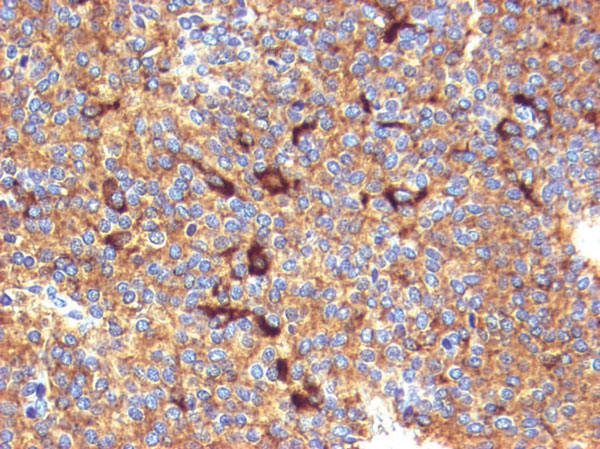
Figure 3. Control gonadotroph adenoma case. Almost all cells are immunoreactive for alpha-SU. Several previously mild to moderate positive cells became strongly immunoreactive after TSA (TSA- EnVision system, original magnification 20×).
Electron microscopic studies:
All adenomas demonstrated poorly developed cytoplasmic membranous organelles including a few scattered lamellar arrays of rough endoplasmic reticulum and Golgi apparatus saccules. The cytoplasm contained sparse and small secretory granules measuring up to 200 nm in some tumors with a tendency to distribution along cytoplasmic membranes. The number and volume density of cytoplasmic mitochondria was variably increased.
DISCUSSION
By definition, adenomas unassociated with signs and clinical symptoms are classified as nonfunctioning or silent; the latter are immunoreactive for pituitary hormones, which are responsible for endocrine syndromes. Up to 30-35% of adenohypophysial tumors are nonfunctioning macroadenomas, currently representing the most common in both clinical and surgical series. They present with mass effect symptoms and hypopituitarism.5 They comprise a heterogeneous group of adenohypophysial tumors, consisting mostly of chromophobic null cell and gonadotroph adenomas. The remaining unusual types include the silent GH-, PRL- and TSH-producing adenomas, the silent corticotroph adenomas subtype 1 and 2 and the rare acidophil stem cell adenoma and silent subtype 3 plurihormonal adenoma.6,7
Null cell and gonadotroph adenomas share similarities in histology and ultrastructure. By histology, they are chromophobic and PAS negative tumors consisting of small ovoid or polar shaped cells, often with perisinusoidal arrangement and pseudo-rosette formation. By electron microscopy they show variably-developed rough endoplasmic reticulum and Golgi apparatus saccules and contain small and sparse secretory granules measuring up to 200nm. Based on the abundance of accumulated mitochondria, they are divided into oncocytic (oncocytomas) and non-oncocytic tumors. However, this distinction is not of clinical relevance.6
The original designation coined by Kovacs et al in 1980 of “null cell” adenomas was based on 56 nonfunctioning tumors among 343 surgically removed pituitary adenomas. Based on the perosidase-antiperoxidase immunoenzymatic (PAP) immunohistochemical method, they contained no adenohypophysial hormones, although electron microscopy showed sparse and small secretory granules.1 However, later studies have definitely shown that null cell adenomas release β-FSH or β-LH in dispersed cell tissue cultures.9,10,11 More advanced molecular studies carried out via Northern blot technique12,13 and by in situ hybridization.14-16 have shown that they may express mRNA of α- and/or β-FSH or β-LH.
Gonadotroph adenomas were thought to be very rare in the past, particularly before application of immunohistochemistry.17,18 In a detailed comparative study of 300 null cell adenomas, oncocytomas and gonadotroph adenomas were analyzed by immunohistochemistry for gonadotropin hormone subunits. It was found that at least 23% of tumors initially diagnosed as null cell adenomas and 28% as oncocytomas could be considered gonadotroph adenomas.8 By electron microscopy, some of these adenomas showed tiny secretory granules along cytoplasmic membranes, often accumulating in the attenuated cytoplasmic processes. These findings, originally described in TSH-secreting adenomas and referred to as “a sign of glycoprotein hormone cell differentiation”, can be observed in gonadotroph adenomas as well. In this study the authors concluded: “...the ever constricted category of null cell adenoma will someday find as little place in the nosology of pituitary adenomas as does the term chromophobic adenoma”. It was therefore speculated that null cell and gonadotroph adenomas belong to the same entity.8
During the last two decades, advanced immunohistochemical techniques utilizing more sensitive detection systems such as ABC have replaced the classic PAP method. This technique, in conjunction with pretreatment with proteolytic digestion to retrieve the hidden antigenic sites due to fixation in formalin, has led to more frequent detection of gonadotropin hormone subunits.8 More recently, the EnVision detection system, a peroxidase-conjugated polymer backbone, which also carries secondary antibody molecules directed against rabbit and mouse immunoglobulins, was introduced. The main advantages of this system include high sensitivity for routine immunohistochemistry and elimination of background staining.18 As a result, among clinically nonfunctioning pituitary adenohypophysial tumors, the diagnosis of null cell adenoma gradually diminished in favor of gonadotroph adenoma.8,18,19 Meanwhile, catalyzed signal amplification systems such as TSA were introduced as a complementary system to improve immunohistochemistry.20 Sanno et al (1996) have demonstrated that the latter technique is extremely sensitive.21,22 In their studies detecting gonadotropin subunits in gonadotroph cells of normal pituitaries, they have noted that catalyzed signal amplification systems are 1000-fold more sensitive than the standard indirect PAP method and 100-fold more than the standard ABC method.22 In the present study, we first applied the TSA technique combined with a secondary polymer antibody containing detection EnVision system to detect pituitary hormones. We were able to detect gonadotropin subunits in all adenomas that were initially completely immunonegative and thus reported as null cell adenomas. Therefore, we were able to reclassify them as gonadotroph adenomas.
In the original paper introducing the term “null cell” adenoma, the authors stated: “…with increasing knowledge, appropriate markers will be found and, with the use of more sophisticated methodology currently unavailable, the number of diagnosed null cell adenoma cases will diminish”.1 The reason that null cell adenomas were negative for gonadotropin hormone subunits is mostly attributed to the low sensitivity of immunohistochemical systems that were not sufficient to detect the low amount of protein hormones stored within the sparse and tiny secretory granules. Therefore, routine immunohistochemistry, even after pretreatment with proteolytic digestion, was not adequate to detect gonadotropin hormone subunits in all cases. Another problem related to the reproducibility of immunohistochemistry is the selection of antibodies used. Obviously, monoclonal antibodies have high specificity but low sensitivity. In contrast, polyclonal antisera are very sensitive, although they might show cross-reactivity for other pituitary hormones. In the current study, given that adenomas were selectively positive for gonadotropin hormone subunits and negative for all remaining pituitary hormones, the problem of specificity is beyond any further discussion.
In conclusion, using currently available advanced and sensitive immunohistochemical protocols, we were able to prove that so-called “null cell” adenomas produce α- or/and β-FSH or β-LH subunits and thus are gonadotroph adenomas in origin. We therefore suggest that the term gonadotroph be applied to any adenoma positive for α-SU or/and β-LH or β-FSH, and negative for GH, PRL, ACTH and β-TSH, irrespective of the number of immunoreactive cells and of staining intensity. However, the term “null cell” adenoma should be reserved for routine diagnosis when immunohistochemistry is negative for all pituitary hormones.
REFERENCES
1. Kovacs K, Ryan N, Horvath E, Ezrin C, 1980 Null cell adenoma of the human pituitary. Virchows Arch [A] Pathol Anat Histopathol 387: 165-174.2. Kontogeorgos G, Kovacs K, Scheithauer BW, Rologis D, Orphanidis G, 1991 Alpha-subunit immunoreactivity in plurihormonal pituitary adenomas of acromegalic patients. Modern Pathol 4: 191-195.
3. Schultz S, Schultz S, Schmitt J, et al, 1998 Immunocytochemical detection of somatostatin receptors SST1, SST2A, SST2B and SST3 in paraffin-embedded breast cancer tissue using subtype-specific antibodies. Clin Cancer Res 4: 2047-2052.
4. Thodou E, Kontogeorgos G, Theodosiou D, Pateraki M, 2006 Mapping of somatostatin receptor types in GH or/and PRL producing pituitary adenomas. J Clin Pathol 59: 274-279.
5. Cooper O, Melmed S, 2012 Subclinical hyperfunctioning pituitary adenomas: The silent tumors. Best Pract Res Clin Endocrinol Metab 26: 447-460.
6. Asa SL 2007 Tumors of the Pituitary Gland. 4th Series - Atlas of Tumor Pathology. American Registry/Armed Forces Institute of Pathology: Washington, DC.
7. DeLellis RA, Heitz P, Lloyd RV, Eng C, (eds) 2007 WHO Classification of Tumours of the Endocrine Organs Pathology and Genetics of Endocrine Organs, IARC Press, Lyon.
8. Kontogeorgos G, Scheithauer BW, Kovacs K, Horvath E, 1993 Null cell adenomas, oncocytomas and gonadotroph adenomas of the human pituitary: An immunocytochemical and ultrastructural analysis of 300 cases. Endocr Pathol 4: 20-29.
9. Asa SL, Gerrie BM, Singer W, Horvath E, Kovacs K, Smyth HS, 1986 Gonadotropin secretion in vitro by human pituitary null cell adenomas and oncocytomas. J Clin Endocrinol Metab 62: 1011-1019.
10. Yamada S, Asa SL, Kovacs K, 1988 Oncocytomas and null cell adenomas of the human pituitary: Morphometric and in vitro functional comparison. Virchows Arch [A] Pathol Anat Histopathol 413: 333-339.
11. Yamada S, Asa SL, Kovacs K, Muller P, Smyth HS, 1989 Analysis of hormone secretion by clinically non functioning human pituitary adenomas using the reverse hemolytic plaque assay. J Clin Endocrinol Metab 66: 73-80.
12. Jameson JL, Klibanski A, Black PM, et al, 1987 Glycoprotein hormone genes are expressed in clinically nonfunctioning pituitary adenomas. J Clin Invest 80: 1472-1478.
13. Sakurai T, Seo H, Yamamoto N, et al, 1988 Detection of mRNA of prolactin and ACTH in clinically nonfunctioning adenomas. J Neurosurg 69: 653-659.
14. Baz E, Saeger W, Uhlig H, Fehr S, Ludecke DK, 1991 HGH, PRL and beta HCG/beta LH gene expression in clinically inactive pituitary adenomas detected by in situ hybridization. Virchows Arch [A] Pathol Anat Histopathol 418: 405-410.
15. Lloyd RV, Jin L, Fields K, et al, 1991 Analysis of pituitary hormones and chromogranin A mRNA in null cell adenomas, oncocytomas and gonadotroph adenomas by in situ hybridization. Am J Pathol 139: 553-556.
16. Schmid M, Münscher A, Saeger W, Schreiber S, Lüdecke DK, 2001 Pituitary hormone mRNA in null cell adenomas and oncocytomas by in situ hybridization comparison with immunohistochemical and clinical data. Pathol Res Pract 97: 663-669.
17. Trouillas J, Girod C, Sassolas G, Caustrat B, 1986 The human gonadotropic adenoma: Pathologic diagnosis and hormonal correlations in 26 tumors. Sem Diagn Pathol 3: 42-57.
18. Vosse BA, Seelentag W, Bachmann A, Bosman F, Yan P, 2007 Background staining of visualization systems in immunohistochemistry: Comparison of the Avidin-Biotin Complex sstem and the EnVision+ System. Appl Immunohistochem Mol Morphol 15: 103-107.
19. Asa SL, Ezat S, Watrson RE Jr, Lindell EP, Horvath E 2007 Gonadotropin producing adenoma. In: DeLellis, RA, Heitz, P, Lloyd RV, Eng C, (eds) WHO Classification of Tumors of the Endocrine Organs: Pathology and Genetics of Endocrine Organs, IARC Press, Lyon, France; pp, 30-32.
20. Borbrow MN, Harris TD, Shaughnessy KJ, Litt GJ, 1989 Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J Immunol Methods 125: 279-285.
21. Sanno N, Teramoto A, Surigiyama M, Itoch Y, Osamura RY, 1996 Application of catalyzed signal amplification in immunodetection of gonadotropin subunits in clinically nonfunctioning pituitary adenomas. Am J Clin Pathol 106: 16-21.
22. Sanno N, Teramoto A, Osamura RY 2001 In: Lloyd RV (ed) Tyramide Amplification: Immunohistochemistry. In: Morphology Methods. Cell and Molecular Biology Techniques, Humana Press, Totowa, New Jersey; pp, 267-276.
Address for correspondence:
George Kontogeorgos, 8 Grinafidi Str. (BLDG#13), 37 300 Agria - Volos, Greece; Tel.: +30 24280-92311 / 6974 377568, Fax: +30 24210-37569, E-mail: gkonto@med.uoa.gr
Received: 21-11-2015, Accepted: 03-01-2016