Institute of Reproductive and Developmental Biology, Department of Surgery and Cancer, Imperial College London, Hammersmith Campus, London, UK; and Department of Physiology, Institute of Biomedicine, University of Turku, Turku, Finland
It is well established in various experimental models that luteinizing hormone (LH) stimulated testosterone (T) production of Leydig cells is the key endocrine stimulus of spermatogenesis. The role of the other gonadotrophin, follicle-stimulating hormone (FSH), is as yet somewhat unclear given that several clinical conditions and experimental models, including men with inactivating FSH receptor (R) mutation and male Fshb and Fshrknockout mice, maintain fairly normal spermatogenesis and fertility. Furthermore, FSH treatment of male infertility has produced at best modest results. On the other hand, there are animal species (e.g. teleost fishes and the Djungarian hamster) where spermatogenesis is primarily FSH-dependent. The purpose of this article is to briefly review the gonadotrophin dependence of spermatogenesis in several model species and examine how it has shifted during evolution from FSH to LH dominance. The information may provide new insight into the role of FSH treatment of male infertility.
Evolution, Follicle-stimulating hormone, Gonadotrophin, Luteinizing hormone, Pituitary gland, Spermatogenesis, Testis
INTRODUCTION
The adult testis has two functions: the production of sex steroids, in particular testosterone (T), and spermatogenesis. Two pituitary gonadotrophic hormones, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), are the pivotal endocrine regulators of these testicular functions. LH stimulates T production by Leydig cells of testicular interstitial tissue. T, on the one hand, is secreted into the circulation where it exerts its e xtragonadal sexual (potency, libido) and anabolic (muscle strength, bone density) actions. On the other hand, T has an intratesticular paracrine role in the maintenance of spermatogenesis, which occurs indirectly through stimulation of Sertoli cells present in the seminiferous tubules adjacent to germ cells. The Sertoli cells then send T-dependent paracrine stimuli to spermatogenic cells. Sertoli cells are also the target of FSH, and thus T and FSH regulate spermatogenesis in a similar indirect paracrine fashion.
While it is textbook knowledge that LH-stimulated T production is quintessential for the maintenance of qualitatively and quantitatively complete spermatogenesis, the specific role of FSH is not to date so clearly delineated. FSH apparently synergizes with T in the regulation of Sertoli cell function, but there are several examples from a variety of mammalian species showing that spermatogenesis can be initiated and maintained with T alone and that sufficient, though quantitatively suppressed, spermatogenesis is possible without FSH. Moreover, considerable species differences exist with respect to the role of FSH in spermatogenesis in comparison with the almost universal dominance of LH/T action.
The purpose of this review is to reflect on the role of FSH in the LH/T-dominated maintenance of spermatogenesis. Evidence will be presented that the involvement of the two gonadotrophins has taken different directions in the course of evolution. We first briefly review the hormonal regulation of spermatogenesis in the standard experimental models, i.e. the laboratory rat and mouse, and humans. Thereafter, some evolutionary milestones in this regulatory process, as well as notable exceptions, are described. This information may help us better understand the pathogenesis of spermatogenic failure in humans and offer novel strategies for the FSH treatment of male infertility.
ROLE OF LH AND T
Rodents
Much of the experimental work on spermatogenesis has been carried out in the two commonest rodent models, the rat and mouse. They can be discussed together because the role of gonadotrophins in the regulation of their spermatogenesis is very similar. Earlier studies were mainly conducted on rats, but the most recent research, in particular genetic manipulation experiments, has been carried out on mice. Rats and mice have demonstrated that T is essential for spermatogenesis but is relatively independent of FSH.
Rat spermatogenesis can be initiated by T treatment alone in animals made gonadotrophin-deficient by immunization against GnRH1 or FSH2,3 or when treated with GnRH antagonist.3 Numerous more recent studies have demonstrated the same LH/T dominance in mouse spermatogenesis (see below).
Before puberty, T is essential for male genital differentiation and growth, including testicular descent and Sertoli cell differentiation. The main steps of rodent spermatogenesis that are under T regulation are meiosis and postmeiotic spermiogenesis. T seems to have no role in spermatogonial proliferation and maturation,4,5 but it supports spermatocyte and spermatid survival in immature rats,6 probably by antiapoptotic mechanisms (summarized by Ruwanpura et al7). Androgen receptor (AR) knockout mice have clearly demonstrated that spermatogenesis does not proceed to complete meiosis without indirect T action through Sertoli cells.8,9
Male Lhb and Lhr knockout mice are azoospermic,10-12 indicating that LH stimulated T production is indispensable for spermatogenesis. However, old Lhr knockout (LuRKO) mice do show low levels of qualitatively complete spermatogenesis, apparently due to the residual LH independent T production of the immature Leydig cells present in LuRKO testes.13 This steroidogenesis is either constitutive or stimulated by paracrine Sertoli cell factors. Because treatment with the antiandrogen flutamide blocks the progression from round to elongating spermatids in old LuRKO mice, the process is clearly shown to be dependent on T stimulation. Although LuRKO mice have elevated FSH levels, due to reduced feedback inhibition of testicular inhibin, the flutamide effect demonstrates that FSH is not able to compensate for the lack of T action in this model.
Numerous studies have demonstrated that FSH alone is not able to rescue full spermatogenesis in gonadotrophin-deficient animals, except for a small increase in the number of spermatogonia and premeiotic spermatocytes;7 however, androgen action is absolutely necessary for the completion of meiosis, progression from round to elongating spermatids and spermiogenesis.14-16
Although Leydig cells produce numerous nonsteroidal paracrine factors, most of the evidence indicates that T is the important mediator of LH effects on spermatogenesis, occurring through paracrine effects of Sertoli cell derived factors. The best evidence for the role of Leydig cell factors other than T in spermatogenesis comes from comparison of T and LH [using the LH agonist human choronic gonadotrophin (hCG)] treatments in gonadotrophin-deficient hpg mice.17 Whereas no difference was found in the production of mature sperm, the number of spermatogonia was larger after hCG treatment. Hence, some Leydig cell factor(s) other than T can promote the proliferation and/or survival of spermatogonia. However, high-dose T supplementation seems to fully restore the number of mature spermatids in LuRKO18,19 and hpg16 mice. Furthermore, transcriptome analysis of testes of wild-type LuRKO and T-treated LuRKO mice showed that most of the defects in gene expression in the absence of LH action were corrected by T treatment.20
Another potential Leydig cell factor stimulating spermatogenesis could be insulin-like factor 3 (INSL3), though it has been shown to be dispensable for mouse spermatogenesis.21 However, recent studies have shown that INSL3 can promote spermatogonial differentiation in zebrafish testis,22,23 providing another example of evolutionary divergence in the hormonal regulation of gametogenesis. The spermatogenic effect of INSL3 thus seems to have been lost in mammals during evolution.
Humans and other primates
The overall LH/T effects on spermatogenesis appear to be rather similar in most mammalian species, thus no major differences exist between rodents and primates in this respect. In humans and monkeys, as in rodents, T is important for the distal part of spermatogenesis, i.e. the conversion of round to elongating spermatids and spermiation.15,24 As in rodents, T also acts as an antiapoptotic survival factor for spermatocytes and spermatids.25
ROLE OF FSH
Rodents
The first actions of FSH on testicular function start in rodents postnatally when Sertoli cells acquire Fshr expression.26 FSH stimulates Sertoli cell proliferation prepubertally and determines their finite number, which then determines the size of the testes and the quantity of spermatogenesis.27 Further evidence for this role of FSH has been obtained from Fshr and Fshb knockout mice, which have somewhat reduced testis size in the face of otherwise roughly normal spermatogenesis and fertility.28-30 Hence, FSH action per se is not necessary for the initiation of spermatogenesis and maintenance of fertility in mice. The main role of FSH is, in synergy with T, to maximize the quantity of sperm production.
In adult rat testis, FSH impacts on gonocyte maturation, acts as an antiapoptotic survival factor for spermatogonia, spermatocytes and spermatids and supports meiosis and spermiogenesis by regulating the adhesion complexes between germ and Sertoli cells (reviewed by Ruwanpura et al7). The findings that FSH treatment increases the numbers of all cell types up to round spermatids may be explained by increased supply of spermatogonia and ‘passive’ increase in subsequent downstream maturational steps, including the meiotic reduction of late pachytene spermatocytes to round spermatids. However, spermatid elongation was not restored by FSH, indicating the need for additional factor(s), most critically T, as described above.31
There is also some evidence of FSH support of spermatocyte survival and of meiosis, but as the sperm mature the role of T becomes more prominent. Sertoli cell-specific AR knockout mice have shown that FSH alone can support mitosis and meiosis during the first spermatogenic wave, but androgen action is absolutely necessary for the completion of meiosis, spermiogenesis and fertility in adult life.8,9
Humans and other primates
The individual role of FSH in human spermatogenesis is still largely unexplored, although many similarities exist between rodents and man. One example comes from men with inactivating FSHR mutation who are subfertile with reduced testis size, but not azoospermic.32 Likewise, male Fshr and Fshb knockout mice are fertile with somewhat suppressed, though qualitatively normal, spermatogenesis (see above). This indicates that, as in rats and mice, human spermatogenesis is mainly LH/T-dependent and FSH is able to synergize with T to assure qualitatively and quantitatively normal spermatogenesis, which however is not an absolute prerequisite for fertility.
Another example of the less crucial role on FSH in human spermatogenesis comes from the classical study where gonadotrophin-suppressed men were treated with hCG, i.e. LH-stimulated T production was achieved in the absence of FSH.33 Near-complete restoration of spermatogenesis was observed, again indicating that the absence of FSH action had only limited suppressive effect on spermatogenesis.
Studies in men with inactivating FSHB or FSHR mutations have yielded controversial results. While the five men described with an inactivating FSHR mutation all have at least some level of spermatogenesis, two of them having sired two children each,32 the three men with inactivating FSHB mutation34-36 were all azoospermic. Of these men two were found in cohorts of azoospermic men, so it is not proven that the FSHB mutation detected was the cause of their phenotype. Only one of the men was detected as a brother of a woman homozygous for the same mutation and with no other abnormality. It is clear that the mutation data for or against the compulsory role of FSH in the onset and maintenance of spermatogenesis in humans are insufficient to allow final conclusions.
In monkeys, the role of FSH in spermatogenesis has long been under debate.37 While there are studies showing that immunization of monkeys for FSH or FSHR brings about azoospermia,38,39 the bulk of evidence shows that the FSH dependence of monkey spermatogenesis is very similar to that of other mammalian species, i.e. FSH is not essential, although it synergizes with T to maximize sperm output.
The overall impression from the existing literature, albeit all findings do not agree, is that the two important roles of FSH in spermatogenesis in rodents and man are to regulate the finite number of Sertoli cells before puberty and to act as a survival factor of premeiotic germ cells. Rather than being a mitogen, FSH is a survival factor constraining apoptosis.
Combined action of FSH and T
Besides its support of proliferation and development of spermatogonia, FSH synergizes with T to support spermiation, i.e. the release of spermatids from Sertoli cells.15 In general terms, although there is plenty of synergy between FSH and T in the regulation of spermatogenesis, the proximal pre-meiotic stages of this process are more FSH-responsive and the post-meiotic maturation is critically T-dependent. Much of this regulation entails the maintenance of balance between cell division and death (apoptosis) where FSH and T act as survival factors.7 The FSH and T effects in the spermatogenic process converge in complementary, synergistic and permissive modes at specific phases of the process to maximize its efficacy (reviewed by7,40,41). One such effect is the regulation of the intrinsic and extrinsic apoptotic pathways of spermatogenic cells.7 Synergism is also observed in spermatocyte development and spermiation.41
FSH and T cooperation is also indicated by findings that lower doses of either hormone is effective when the other one is present.42-44 This view has led to the speculation that T and FSH may have common postreceptor pathways of action,45 evidence for which is still lacking, especially since the main signaling pathway evoked by FSHR activation, i.e. protein kinase A-cyclic AMP, is not stimulated by T. However, there are additional signaling systems, like MAP kinase and CREB, where FSH and T actions can converge.46
It seems that the role of FSH in mammalian spermatogenesis is less important, since there are many models where spermatogenesis and fertility are maintained at sufficient levels without FSH action. One can therefore question the necessity and meaning of such a weak stimulus of spermatogenesis. Current knowledge of the evolution of the two-gonadotrophin system could offer an explanation, as will be discussed below.
SPECIES DIFFERENCES: EVOLUTIONARY ASPECTS
Concerning the building blocks of gonadotrophin function, i.e. the expression of growth factors with cysteine knot structural signature, such as TGFb and glycoprotein hormones (GPHs), including gonadotrophins, their genes are found in the genome of almost all metazoan (multicellular animals) with the exception of very primitive forms like the sea anemone and sponge.47 Likewise, leucin-rich repeat-containing G protein-coupled receptors (GPCRs), akin to gonadotrophin receptors in higher species, have been detected in basal metazoans.47,48 It is therefore possible that gonadotrophin action-like regulation of reproduction is a very archaic early evolutionary event, present in the most primitive metazoans. Whether gonadotrophin regulation of spermatogenesis starts with FSH and/or LH action is crucially dependent on the divergence of the two gonadotrophins and their receptors during evolution.
Lower animal species
The most ancestral form of GPHs is thyrostimulin whose homologues can be detected both in vertebrates and invertebrates, including the fly, nematode and sea urchin,49,50 but not in basal animals such as the sea anemone.47 It has α and β subunits like all GPHs, which have arisen through gene duplication of a single ancestral gene.51 These subunits have given rise, through another whole-genome duplication, to another GPH, which is first detected as the single gonadotrophin in the most primitive ancestral agnathan (jawless) vertebrates.52-54 Hagfish (or the slime eel) and lampreys are the two remaining members of this group and considered the most primitive vertebrates known, living or extinct. Their single pituitary GPH has no specific LH- or FSH-like function. The next evolutionary step includes retention of thyrostimulin in vertebrates and the divergence of hagfish GTHb to Lhb, Fshb and Tshb during additional whole-genome duplications. The first duplication product was akin to Lhb, after which the common ancestor of Fshb and Tshb formed during the next duplication.51 As a result of this, most jawed teleost fishes have two gonadotrophins, Lh and Fsh, akin to those in mammals.55 However, the biological actions of the two primitive fish gonadotrophins are not as well-defined as in mammals.
Two glycoprotein hormone receptors, again results of gene duplication and functional diversification, are first encountered in the lamprey, but it has not been possible to classify them unequivocally as being specific for Lh, Fsh or Tsh.56 Their overall structure is surprisingly close to their mammalian counterparts. Interestingly, despite the two gonadotrophin receptors, the lamprey has only one gonadotrophin. It is therefore understandable that the two lamprey gonadotrophin receptors cannot have Lhr- or Fshr-like specificity because these two ligands only evolve later. It therefore seems that the coevolution of the two-gonadotrophin system is driven by the evolution of receptors, which is followed by that of the cognate ligands.
Teleost fish are the first evolutionary stage that clearly has two gonadotrophins and their cognate receptors with partially acquired specificity. Although there are exceptions, the general pattern in fishes is that Fshr is responsive to both Fsh and Lh, whereas Lhr only responds to Lh.55 Besides functional promiscuity, in addition the sites of expression of fish gonadotrophin receptors are more relaxed than will be the case with higher vertebrates. Taking into account the earlier appearance of Lh (see above), the fact that Lh can activate both Lhr and Fshr and that the gonadotrophin-responsive gonadal cells initially expressed both gonadotrophin receptors, it is enigmatic why evolution took the path of introducing the two-gonadotrophin system for gonadal regulation. The Lh/Lhr system might have been sufficient, as is implied by the minor role of Fsh in male mammalian fertility (see above). The two gonadotrophins were mainly created for the needs of ovarian function.
Zebrafish
It appears that teleost fishes, including the model species zebrafish, are halfway through in the evolution of the specific two-gonadotrophin regulatory system of gonadal function. As described before, lampreys and hagfish have one gonadotrophin and two non-specific gonadotrophin receptors. Teleost fishes have acquired two specific gonadotrophins, but their cognate receptors still possess some degree of promiscuity: Fshr responds to both Fsh and Lh, whereas only Lhr is specific for the cognate ligand.57 Another divergence from the mammalian system is that Fshr and Lhr are expressed in fishes both in Leydig and Sertoli cells.58,59
Recent studies on gonadotrophin and gonadotrophin receptor knockouts in zebrafish using the TALE technique60-62 have elucidated the marked special features of gonadotrophin action that diverge from mammals. Neither lhb nor lhr knockouts affected testicular histology, fertilization rate or sperm motility in male fishes, indicating that Lh signaling, unlike in mammals, is not important for the fertility of the male zebrafish. This would indicate the importance of Fsh action for male zebrafish fertility. Nevertheless, fshb- and fshr-deficient male zebrafish were fertile, with only some delay in sexual maturation. The promiscuity of Fshr to Lh and Fsh stimulation and the coexpression of both gonadotrophin receptors in Leydig and Sertoli cells provide an explanation for these apparently surprising findings. Double lhb/fshb knockout males showed delayed sexual maturation but were finally fertile. Infertility with azoospermia was only found in lhr/fshr double knockout males.
The studies show that unlike in mammals, Lh action is not needed for zebrafish spermatogenesis and is more dependent on Fshr activation. However, in the absence of Fsh, Lh can activate Fshr, and in the absence of Fshr its effect is compensated for by Lh action because both gonadotrophin receptors are expressed in Leydig and Sertoli cells. Finally, the mild phenotype of combined lhb/fshb deletion can be explained by the fact that non-liganded gonadotrophin receptors may have a low level of constitutive activity which is able to maintain a low level of Lhr/Fshr activity sufficient to maintain partial spermatogenesis. A similar difference is observed in male mice where the testicular phenotype is more severe in Fshr than Fshb knockout63 apparently for the same reason.
When we now consider the evolution of gonadotrophin maintenance of spermatogenesis, it seems that the specialization of LH and FSH action has occurred gradually and taken a different course in various species. First, jawless fishes have one gonadotrophin and two non-specific gonadotrophin receptors. Thereafter, the two gonadotrophins appear in bony fishes, which show a relaxed specificity in ligand-receptor interaction. Usually only LHR is specific for the cognate hormone and FSH is the dominant gonadotrophin in spermatogenesis. The situation will be the opposite in mammals, but with some remarkable exceptions.
The Djungarian hamster
The Djungarian hamster (Phodopus sungorus), a seasonally breeding rodent, forms a peculiar exception in the mode of hormonal control of spermatogenesis amongst mammalian species. Hypophysectomized males or those gonadotrophin-suppressed during short days (photoinhibited) respond to FSH treatment with full recovery of spermatogenesis without simultaneous stimulation of Leydig cell steroidogenesis.64-66 In contrast, LH alone has a marginal effect on spermatogenesis even though it restores testicular T production to control level. It seems therefore that FSH alone, and without additive or synergistic action of T, is able to drive the whole spermatogenic process in this species. Remarkably, no effect of FSH on Leydig cell function, including stimulation of T production, could be detected. Furthermore, T synergized with FSH in the maintenance of spermatogenesis in the Djungarian hamster because sperm production, and in particular the number of elongating spermatids, was higher in the combined treatment than with FSH alone.64 This finding emphasizes the role of T in the maturation step from round to elongating spermatids, also considered crucial in rodents.
Further studies in photoinhibited and antiandrogen (flutamide) treated animals demonstrated that FSH treatment in these conditions was able to increase Sertoli cell and spermatogonial numbers and to advance the premeiotic phases of spermatogenesis.67 The conditions used were not appropriate to study whether FSH would have increased the numbers of postmeoiotic germ cells, which leaves the question open whether FSH in the absence of T is able to advance later stages of spermatogenesis. Because a low level of T production is possible in the photoinhibited hamster testis in the absence of LH stimulation, the current evidence is not sufficient to conclude whether postmeiotic maturation of hamster sperm is possible with FSH stimulation alone. The least we can conclude about the Djungarian hamster is that its spermatogenesis is predominantly dependent on FSH with a possible supporting role of T, which is the opposite to other mammalian species.
PHARMACOLOGICAL AND GENETIC AMPLIFICATION OF FSH ACTION: EFFECTS ON SPERMATOGENESIS
The efficacy of FSH therapy in the treatment of oligozoospermia remains a contentious issue.68-70 The results are variable, although there are some promising new leads indicating that specific genetic polymorphisms in FSHb and FSHR may cause functional FSH deficiency, in which case FSH therapy may improve spermatogenesis.71-73 To be effective, FSH therapy will therefore require stratification of men according to genotype into FSH-responsive and non-responsive individuals.
Another explanation for the poor outcome of the FSH treatments is that the doses used have been too small, and to be effective they have to be pharmacological. Paradisi et al74,75 and Ding et al76 have observed in placebo-controlled studies that a high dose of recombinant FSH (300 IU every other day for ≥4-5 months), instead of the standard dose of 75 IU/every other day, significantly increased sperm counts and pregnancy rates.
The success of treatment with higher FSH doses may have relevance to some additional findings in the involvement of FSH in spermatogenesis. Gromoll et al reported in 199677 a hypophysectomized male who had normal spermatogenesis despite unmeasurable gonadotrophin levels. He was found to carry a D567G mutation in exon 10 of FSHR, which evoked marginal constitutive activity of FSHR. Replication of the same mutation in transgenic mice in the gonadotrophin-deficient hpg background increased the number of FSH-responsive Sertoli cells, spermatogonia and postmeiotic germ cells, but did not lead to full spermatic maturation. Hence, although the mouse model confirmed the constitutive biological activity of the human D567G FSHR mutation, it did not confirm the human finding of full spermatogenesis. Furthermore, these models were confounded by the paracrine stimulation of Leydig cell T production by the FSH-stimulated Sertoli cells, which made it difficult to decipher whether the spermatogenic effect was due to this effect or direct Sertoli cell stimulation by the mutant FSHR. Our very recent findings (Oduwole et al, manuscript in preparation) of fully compensated spermatogenesis in another genetically modified mouse model of a strongly activating Fshr mutation expressed in the Lhr-/- background suggests that the spermatogenesis in the male patient may really be due to the FSHR mutation and that the lack of a full phenocopy in the mouse may be due to too weak activity of the human gain-of-function FSHR mutation in the mouse.
Taken together, these findings suggest that very strong FSHR stimulation can compensate for the lack of androgen stimulation in spermatogenesis and that T and FSH can totally compensate for each other, provided that the level of stimulation of the remaining hormone is strong enough. Hence, there seem to be no specific stages of spermatogenesis that are exclusively T- or FSH-dependent. The dose may be critical when treating oligozoospermia with FSH and the typically used doses may have been insufficient. Finally, the currently accepted assumption that T is a ‘sine qua non’ in spermatogenesis may not be true. Almost the same can be achieved with FSH, but it requires pharmacological doses.
CONCLUDING REMARKS
In this review we have made an evolutionay journey from lamprey to humans in the gonadotrophin regulation of spermatogenesis (Figure 1). The regulation starts from the action of a single gonadotrophin on two non-specific receptors in the slime eel and lamprey, and moves to jawed fishes in which FSH is the dominant gonadotrophin in a situation where FSHR has relaxed ligand specificity to FSH and LH and where both gonadotrophin receptors are expressed in Sertoli and Leydig cells. When evolution reaches mammals, LHR and FSHR have become specific for their cognate ligands and are expressed only in Leydig or Sertoli cells, respectively. FSH can still in a paracrine fashion, through Sertoli cells, stimulate some T production, but in mammals in general LH is the driving force of spermatogenesis. The Djungarian hamster forms a remarkable exception that proves the rule, maintaining the fish-like FSH-dependent pattern of spermatogenic regulation. The real importance of FSH in the regulation of mammalian spermatogenesis still remains an enigma, since many mammalian models seem to maintain sufficient spermatogenesis in the absence of FSH. The effect of strong FSH stimulation on mammalian spermatogenesis appears to hark back to the evolutionarily earlier fish and suggests that higher than previously presumed doses of FSH may be effective in the treatment of male infertility.
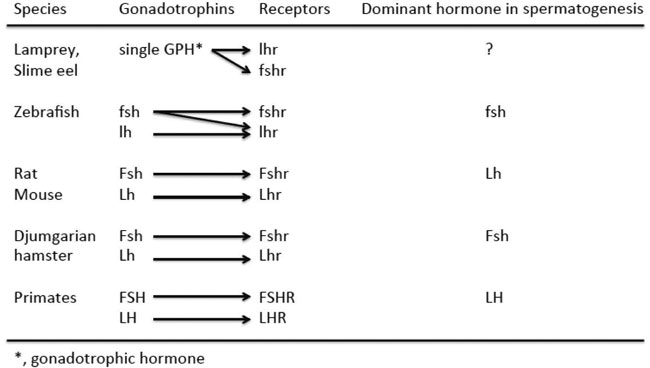
Figure 1. The gonadotrophins, their receptors, specificity of the interaction and dominant gonadotrophin in the regulation of spermatogenesis in the species discussed in the text. The abbreviations of the molecules are according to the Committee on Genetic Symbols and Nomenclature (1957). Union of International Sci Biol Ser B, Colloquia No. 30.
REFERENCES
1. Awoniyi C A, Santulli R, Chandrashekar V, Schanbacher BD, Zirkin BR, 1989 Quantitative restoration of advanced spermatogenic cells in adult male rats made azoospermic by active immunization against luteinizing hormone or gonadotropin-releasing hormone. Endocrinology 125: 1303-1309.
2. Madhwa Raj HG, Dym M, 1976 The effects of selective withdrawal of FSH or LH on spermatogenesis in the immature rat. Biol Reprod 14: 489-494.
3. Rivier C, Rivier J,Vale W, 1981 Effect of a potent GnRH antagonist and testosterone propionate on mating behavior and fertility in the male rat. Endocrinology 108: 1998-2001.
4. Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM, 2003 Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology 144: 509-517.
5. Holdcraf RW, Braun RE, 2004 Hormonal regulation of spermatogenesis. Int J Androl 27: 335-342.
6. Tapanainen JS, Tilly JL, Vihko KK, Hsueh AJ, 1993 Hormonal control of apoptotic cell death in the testis: gonadotropins and androgens as testicular cell survival factors. Mol Endocrinol 7: 643-650.
7. Ruwanpura SM, McLachlan RI, Meachem SJ, 2010 Hormonal regulation of male germ cell development. J Endocrinol 205: 117-131.
8. Chang C, ChenYT, Yeh SD, et al, 2004 Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A 101: 6876-6881.
9. De Gendt K, Swinnen JV, Saunders PT, et al, 2004 A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A 101: 1327-1332.
10. Lei, ZM, Mishra S, Zou W, et al, 2001 Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol Endocrinol 15: 184-200.
11. Ma X, Dong Y, Matzuk MM, Kumar TR, 2004 Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci U S A 101: 17294-17299.
12. Zhang FP, Poutanen M, Wilbertz J, Huhtaniemi I, 2001 Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol Endocrinol 15: 172-183.
13. Zhang FP, Pakarainen T, Poutanen M, Toppari J, Huhtaniemi I, 2003 The low gonadotropin-independent constitutive production of testicular testosterone is sufficient to maintain spermatogenesis. Proc Natl Acad Sci U S A 100: 13692-13697.
14. Allan CM, Garcia A, Spaliviero J, et al, 2004 Complete Sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: evidence from genetic models of isolated FSH action. Endocrinology 145: 1587-1593.
15. McLachlan RI, O’Donnell L, Meachem SJ, et al, 2002 Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res 57:149-179.
16. Singh J, O’Neill C, Handelsman DJ, 1995 Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology 136: 5311-5321.
17. Spaliviero JA, Jimenez M, Allan CM, Handelsman DJ, 2004 Luteinizing hormone receptor-mediated effects on initiation of spermatogenesis in gonadotropin-deficient (hpg) mice are replicated by testosterone. Biol Reprod 70: 32-38.
18. Oduwole OO, Vydra M, Wood NE, et al, 2014 Overlapping dose responses of spermatogenic and extragonadal testosterone actions jeopardize the principle of hormonal male contraception. FASEB J 28: 2566-2576.
19. Pakarainen T, Zhang ZP, Mäkelä S, Poutanen M, Huhtaniemi I, 2005 Testosterone replacement therapy induces spermatogenesis and partially restores fertility in luteinizing hormone receptor knockout mice. Endocrinology 146: 596-606.
20. Griffin DK, Ellis PJ, Dunmor B, et al, 2010 Transcriptional profiling of luteinizing hormone receptor-deficient mice before and after testosterone treatment provides insight into the hormonal control of postnatal testicular development and Leydig cell differentiation. Biol Reprod 82: 1139-1150.
21. Huang Z, Rivas B, Agoulnik AI, 2012 Insulin-like 3 signaling is important for testicular descent but dispensable for spermatogenesis and germ cell survival in adult mice. Biol Reprod 87: 143, 1-8.
22. Assis LH, Crespo D, Morais RD, Franca LR, Bogerd J, Schulz RW, 2015 INSL3 stimulates spermatogonial differentiation in testis of adult zebrafish (Danio rerio). Cell Tissue Res [Epub ahead of print].
23. Nobrega RH, de Souza Morais RD, Crespo D, et al, 2015 Fsh stimulates spermatogonial proliferation and differentiation in zebrafish via Igf3. Endocrinology 156: 3804-3817.
24. Matthiesson KL, McLachlan RI, O’Donnell L, et al, 2006 The relative roles of follicle-stimulating hormone and luteinizing hormone in maintaining spermatogonial maturation and spermiation in normal men. J Clin Endocrinol Metab 91: 3962-3969.
25. Vera Y, Erkkilä K, Wang C, et al, 2006 Involvement of p38 mitogen-activated protein kinase and inducible nitric oxide synthase in apoptotic signaling of murine and human male germ cells after hormone deprivation. Mol Endocrinol 20: 1597-1609.
26. Rannikki AS, Zhang FP, Huhtaniemi IT, 1995 Ontogeny of follicle-stimulating hormone receptor gene expression in the rat testis and ovary. Mol Cell Endocrinol 107: 199-208.
27. Sharpe RM, McKinnell C, Kivlin C, Fisher JS, 2003 Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125: 769-784.
28. Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM, 2000 The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 141: 1795-1803.
29. Dierich A, Sairam MR, Monaco L, et al, 1998 Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A 95: 13612-13617.
30. Kumar TR, Wang Y, Lu N, Matzuk MM, 1997 Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15: 201-204.
31. McLachlan RI, Wreford NG, de Kretser DM, Robertson DM, 1995 The effects of recombinant follicle-stimulating hormone on the restoration of spermatogenesis in the gonadotropin-releasing hormone-immunized adult rat. Endocrinology 136: 4035-4043.
32. Tapanainen JS, Aittomäki K, Min J, Vaskivuo T, Huhtaniemi IT, 1997 Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet 15: 205-206.
33. Bremner WJ, Matsumoto AM, Sussman AM, Paulsen CA, 1981 Follicle-stimulating hormone and human spermatogenesis. J Clin Invest 68: 1044-1052.
34. Layman LC, Lee EJ, Peak DB, et al, 1997 Delayed puberty and hypogonadism caused by mutations in the follicle-stimulating hormone beta-subunit gene. N Engl J Med 337: 607-611.
35. Lindstedt G, Nyström E, Matthews C, Ernest I, Janson PO, Chatterjee K, 1998 Follitropin (FSH) deficiency in an infertile male due to FSHbeta gene mutation. A syndrome of normal puberty and virilization but underdeveloped testicles with azoospermia, low FSH but high lutropin and normal serum testosterone concentrations. Clin Chem Lab Med 36: 663-665.
36. Phillip M, Arbelle JE, Segev Y, Parvari R, 1998 Male hypogonadism due to a mutation in the gene for the beta-subunit of follicle-stimulating hormone. N Engl J Med 338: 1729-1732.
37. Plant TM, Marshall GR, 2001 The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev 22: 764-786.
38. Moudgal NR, Murthy GS, Prasanna Kumar PS, et al, 1997 Responsiveness of human male volunteers to immunization with ovine follicle stimulating hormone vaccine: results of a pilot study. Hum Reprod 12: 457-463.
39. Moudgal NR, Ravindranath N, Murthy GS, Dighe RR, Aravindan GR, Martin F, 1992 Long-term contraceptive efficacy of vaccine of ovine follicle-stimulating hormone in male bonnet monkeys (Macaca radiata). J Reprod Fert 96: 91-102.
40. McLachlan RI, O’Donnell L, Meachem SJ, et al, 2002 Hormonal regulation of spermatogenesis in primates and man: insights for development of the male hormonal contraceptive. J Androl 23: 149-162.
41. Saito K, O’Donnell L, McLachlan RI, Robertson DM, 2000 Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology 141: 2779-2785.
42. Bartlett JM, Weinbauer GF, Nieschlag E, 1989 Differential effects of FSH and testosterone on the maintenance of spermatogenesis in the adult hypophysectomized rat. J Endocrinol 121: 49-58.
43. Sun YT, Irby DC, Robertson DM, de Kretser DM, 1989 The effects of exogenously administered testosterone on spermatogenesis in intact and hypophysectomized rats. Endocrinology 125: 1000-1010.
44. Sun YT, Wreford NG, Robertson DM, de Kretser DM, 1990 Quantitative cytological studies of spermatogenesis in intact and hypophysectomized rats: identification of androgen-dependent stages. Endocrinology 127: 1215-1223.
45. Russell LD, Corbin TJ, Borg KE, De Franca LR, Grasso P, Bartke A, 1993 Recombinant human follicle-stimulating hormone is capable of exerting a biological effect in the adult hypophysectomized rat by reducing the numbers of degenerating germ cells. Endocrinology 133: 2062-2070.
46. Walker WH, Cheng J, 2005 FSH and testosterone signaling in Sertoli cells. Reproduction 130: 15-28.
47. Roch GJ, Sherwood NM, 2014 Glycoprotein hormones and their receptors emerged at the origin of metazoans. Genom Biol Evol 6: 1466-1479.
48. Nothacker HP, Grimmelikhuijzen CJ, 1993 Molecular cloning of a novel, putative G protein-coupled receptor from sea anemones structurally related to members of the FSH, TSH, LH/CG receptor family from mammals. Biochem Biophys Res Commun 197: 1062-1069.
49. Park JI, Semyonov J, Chang CL, Hsu SY, 2005 Conservation of the heterodimeric glycoprotein hormone subunit family proteins and the LGR signaling system from nematodes to humans. Endocrine 26: 267-276.
50. Sudo S, Kuwabara Y, Park JI, Hsu SY, Hsueh AJ, 2005 Heterodimeric fly glycoprotein hormone-alpha2 (GPA2) and glycoprotein hormone-beta5 (GPB5) activate fly leucine-rich repeat-containing G protein-coupled receptor-1 (DLGR1) and stimulation of human thyrotropin receptors by chimeric fly GPA2 and human GPB5. Endocrinology 146: 3596-3604.
51. Li MD, Ford JJ, 1998 A comprehensive evolutionary analysis based on nucleotide and amino acid sequences of the alpha- and beta-subunits of glycoprotein hormone gene family. J Endocrinol 156: 529-542.
52. Nozaki M, 2013 Hypothalamic-pituitary-gonadal endocrine system in the hagfish. Front Endocrinol (Lausanne) 4: 200, 1-8.
53. Sower SA, Freamat M, Kavanaugh SI, 2009 The origins of the vertebrate hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) endocrine systems: new insights from lampreys. Gen Comp Endocrinol 161: 20-29.
54. Uchida K, Moriyama S, Chiba H, et al, 2010 Evolutionary origin of a functional gonadotropin in the pituitary of the most primitive vertebrate, hagfish. Proc Natl Acad Sci U S A 107: 15832-15837.
55. Levavi-Sivan B, Bogerd J, Mananos EL, Gomez A, Lareyre JJ, 2010 Perspectives on fish gonadotropins and their receptors. Gen Comp Endocrinol 165: 412-437.
56. Freamat M, Sower SA, 2010 Functional divergence of glycoprotein hormone receptors. Integr Comp Biol 50: 110-123.
57. Bogerd J, Granneman JC, Schulz RW, Vischer HF, 2005 Fish FSH receptors bind LH: how to make the human FSH receptor to be more fishy? Gen Comp Endocrinol 142: 34-43.
58. Garcia-Lopez A, de Jonge H, Nobrega RH, et al, 2010 Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins. Endocrinology 151: 2349-2360.
59. Planas JV, Swanson P, 1995 Maturation-associated changes in the response of the salmon testis to the steroidogenic actions of gonadotropins (GTH I and GTH II) in vitro. Biol Reprod 52: 697-704.
60. Chu L, Li J, Liu Y, Hu W, Cheng CH, 2014 Targeted gene disruption in zebrafish reveals noncanonical functions of LH signaling in reproduction. Mol Endocrinol 28: 1785-1795.
61. Zhang Z, Lau SW, Zhang L, Ge W, 2015 Disruption of zebrafish follicle-stimulating hormone receptor (fshr) but not luteinizing hormone receptor (lhcgr) gene by TALEN leads to failed follicle activation in females followed by sexual reversal to males. Endocrinology 56: 3747-3762.
62. Zhang Z, Zhu B, Ge W, 2015 Genetic analysis of zebrafish gonadotropin (FSH and LH) functions by TALEN-mediated gene disruption. Mol Endocrinol 29: 76-98.
63. Baker PJ, Pakarinen P, Huhtaniemi IT, et al, 2003 Failure of normal Leydig cell development in follicle-stimulating hormone (FSH) receptor-deficient mice, but not FSHbeta-deficient mice: role for constitutive FSH receptor activity. Endocrinology 144: 138-145.
64. Lerchl, A, Sotiriadou S, Behre HM, et al, 1993 Restoration of spermatogenesis by follicle-stimulating hormone despite low intratesticular testosterone in photoinhibited hypogonadotropic Djungarian hamsters (Phodopus sungorus). Biol Reprod 49: 1108-1116.
65. Milette JJ, Schwartz NB, Turek FW, 1988 The importance of follicle-stimulating hormone in the initiation of testicular growth in photostimulated Djungarian hamsters. Endocrinology 122: 1060-1066.
66. Niklowitz P, Khan S, Bergmann M, Hoffmann K, Nieschlag E, 1989 Differential effects of follicle-stimulating hormone and luteinizing hormone on Leydig cell function and restoration of spermatogenesis in hypophysectomized and photoinhibited Djungarian hamsters (Phodopus sungorus). Biol Reprod 41: 871-880.
67. Meachem SJ, Stanton PG, Schlatt S, 2005 Follicle-stimulating hormone regulates both Sertoli cell and spermatogonial populations in the adult photoinhibited Djungarian hamster testis. Biol Reprod 72: 1187-1193.
68. Attia AM, Abou-Setta AM, Al-Inany HG, 2013 Gonadotrophins for idiopathic male factor subfertility. Cochrane Database Syst Rev 8: CD005071.
69. Rastrelli G, Corona G, Mannucci E, Maggi M, 2014 Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology 2: 794-808.
70. Valenti D, La Vignera S, Condorelli RA, et al, 2013 Follicle-stimulating hormone treatment in normogonadotropic infertile men. Nat Rev Urol 10: 55-62.
71. Ferlin A, Foresta C, 2014 New genetic markers for male infertility. Curr Opin Obstetr Gynecol 26: 193-198.
72. Selice R, Garolla A, Pengo M, Caretta N, Ferlin A, Foresta C, 2011 The response to FSH treatment in oligozoospermic men depends on FSH receptor gene polymorphisms. Int J Androl 34: 306-312.
73. Tüttelmann F, Laan M, Grigorova M, Punab M, Sober S, Gromoll J, 2012 Combined effects of the variants FSHB -211G>T and FSHR 2039A>G on male reproductive parameters. J Clin Endocrinol Metab 97: 3639-3647.
74. Paradisi R, Busacchi P, Seracchioli R, Porcu E, Venturoli S, 2006 Effects of high doses of recombinant human follicle-stimulating hormone in the treatment of male factor infertility: results of a pilot study. Fertil Steril 86: 728-731.
75. Paradisi R, Natali F, Fabbri R, Battaglia C, Seracchioli R, Venturoli S, 2014 Evidence for a stimulatory role of high doses of recombinant human follicle-stimulating hormone in the treatment of male-factor infertility. Andrologia 46: 1067-1072.
76. Ding YM, Zhang XJ, Li JP, et al, 2015 Treatment of idiopathic oligozoospermia with recombinant human follicle-stimulating hormone: a prospective, randomized, double-blind, placebo-controlled clinical study in Chinese population. Clin Endocrinol [Epub ahead of print]
77. Gromoll J, Simoni M, Nieschlag E, 1996 An activating mutation of the follicle-stimulating hormone receptor autonomously sustains spermatogenesis in a hypophysectomized man. J Clin Endocrinol Metab 81: 1367-1370.
Address for correspondence:
Ilpo Huhtaniemi, Institute of
Reproductive and Developmental Biology, Department of Surgery and Cancer,
Imperial College London, Hammersmith Campus, Du Cane Road, London W12 0NN, UK;
and Department of Physiology, Institute of Biomedicine, University of Turku,
Kiinamyllynkatu 10, 20520 Turku, Finland; E-mail:
ilpo.huhtaniemi@imperial.ac.ukt