1Department of Endocrinology and Diabetes Center, “G. Gennimatas”, General Hospital of Athens; 2Department of Hygiene, Epidemiology and Medical Statistics, National and KapodistrianUniversity of Athens Medical School; Athens, Greece
OBJECTIVE: To monitor and control the blood glucose levels in
inefficiently insulin-treated patients with type 1 and 2 diabetes mellitus (DM)
using a telemonitoring system and determine whether the improvement of HbA1c
has a lasting effect following its discontinuation.
DESIGN: Seventy
inefficiently controlled insulin-treated DM patients using telemonitoring
(telemonitoring group-TG) [HbA1c 9.9±2.3% (85±24.9mmol/mol)] and 35 age-, body mass index
(BMI)- and Hba1c-matched insulin-treated patients receiving outpatient care
(control group-CG) [HbA1c 9.7±2.1% (82±23.4mmol/mol)] were enrolled. Data of TG
were transmitted from the glucose-meters to our computers via modem.
Communication was achieved via e-mails and mobile phone text-messages through
integrated software. HbA1c and BMI were evaluated at enrollment, 3 and 6
months, and 6 months after telemonitoring discontinuation. Frequency of hypo-
and hyperglycemias and cost were also analyzed.
RESULTS: Significant reduction
in HbA1c was observed in TG both at 3 [7.1±1.0% (54±10.5mmol/mol) p<0.001]
and 6 months [6.9±0.9% (52±9.5mmol/mol) p<0.001], compared to the CG group
at the same timepoints. Significant reduction was also observed in the TG
subgroups with ΗbA1c≥10% and 10>HbA1c≥7.5% at 3 and 6 months, compared to
CG. No statistically significant differences in BMI were observed between TG
and CG. Six months after telemonitoring discontinuation, HbA1c in TG was
slightly increased [7.3±1.0% (56±10.4mol/mol)]. Attenuation was also observed
in both TG subgroups. Compared to CG, the number of monthly hypo- and
hyperglycemias was reduced in TG. The intervention had a financial benefit for
patients living more than 100 km from the health care provider.
CONCLUSIONS:
Telemonitoring can result in reduction of HbA1c and frequency of hypo- and
hyperglycemias. This beneficial effect is slightly attenuated 6 months after
terminating telemonitoring.
BMI, Cost, Diabetes, HbA1c, Telemedicine, Telemonitoring
INTRODUCTION
Diabetes mellitus (DM) has emerged as one of the most challenging health problems in the 21st century, today reaching epidemic proportions throughout the world. Type 2 DM patients are expected to rise to 592 million globally by 2035, while there are also almost 500,000 children aged under 15 years with type 1 DM worldwide.1,2 Compared with the data of previous IDF Diabetes Atlas editions, an increased DM incidence rate is documented in many countries.3 In addition, approximately 45.8% of all adult DM cases are estimated to be undiagnosed.4
DM imposes significant social and economic burdens, since increased glycosylated hemoglobin (HbA1c) is associated with high morbidity and mortality rates due to multiorgan complications.5 Tight glycemic control can reduce microvascular complications in both type 1 and type 2 DM and, if implemented early in the course of DM, it appears to significantly reduce the risk of subsequent macrovascular complications.6-10
Despite the availability of several effective therapies to improve HbA1c, many patients fail to achieve DM control. Inadequate outpatient services, a shortage of specialized personnel, geographical isolation, financial hardship, psychological implications, suboptimal glucose monitoring and lifestyle changes in conjunction with inadequate patient education are some of the factors preventing regular follow-up and therapy compliance. Type 1 DM patients are usually children or young adults who frequently struggle with DM self-management, anxiety and depression, especially at the onset of the disease.11,12 On the other hand, type 2 DM patients are usually older individuals who find it hard to alter established habits regarding exercise and nutrition, while comorbidities may complicate glycemic control. Finally, there are certain patient groups (mentally ill, disabled or elderly institutionalized patients) in whom optimal glycemic control is not always feasible. Since aging populations, personnel shortage and reduced financial resources are affecting health systems worldwide, new ways to manage DM should be implemented.
Given the enormous advances seen over the last 30 years in telecommunications and informatics technology, it is evident that the present-day widespread use of the mobile telephone could be exploited to improve DM management. Telemedicine includes the use of telecommunication and electronic information processing technologies [audio, video, short message service (SMS), multimedia messaging service (MMS), internet applications] for remotely monitoring (telemonitoring), diagnosing, treating, supporting and/or consulting patients who live at a large distance from the health care provider. Telemonitoring is based on an automated process for the transmission of medical data on a patient’s status from his/her residence to the respective health care institution. Telemonitoring could improve DM management, prevent hospitalizations due to DM complications and overcome geographical isolation, thus saving time and financial resources.
So far, several studies have been performed on the effect of telemonitoring on DM regulation. Strategies implemented included the use of videoconferencing, modem data transmission, internet-based applications, telephone calls and mobile phone information exchange through SMS. However, only selected RCTs have met the requirements (i.e patient number, both DM types, duration, active intervention following real-time data transmission) for performing proper stratified meta-analyses.13-28 Moreover, duration of telemonitoring effect and cost-effectiveness have not been systematically addressed.29,30
The aim of the present study was to compare the effects of telemonitoring on HbA1c, body mass index (BMI), frequency of hyper- and hypoclycemias and cost of insufficiently controlled insulin-treated DM patients versus usual outpatient care alone. The duration of HbA1c improvement has also been studied in order to determine any potential long-standing beneficial effects of the intervention.
SUBJECTS AND METHODOLOGY
Patients
We conducted a prospective, randomized, controlled trial comparing HbA1c levels and BMIs among a group of insulin-treated DM patients participating in a diabetes telemonitoring program and a group of insulin-treated DM patients receiving usual outpatient care at the Department of Endocrinology. Eligible patients were type 1 and type 2 DM individuals, aged 15 years and older, receiving any kind of insulin treatment, with or without additional oral glucose-lowering agents, and insufficient DM control with a 7.5≤HbA1c<10% (58≤HbA1c<86 mmol/mol) despite at least two years of follow-up at the outpatient department. Additional inclusion criterion was recent hospitalization for newly diagnosed insulin-treated DM in conjunction with HbA1c ≥10% (86 mmol/mol). Exclusion criteria were: acute uncontrolled mental illness and poor eyesight. The nature of the intervention prevented blinding of either patients or physicians. However, the investigator who evaluated outcomes was unconnected to the study and unaware of intervention assignments and grouping. The study was registered in the Australian New Zealand Clinical Trial Registry (ACTRN12614000977673) and the reporting of the study conformed to the Consolidated Standards of Reporting Trials (CONSORT) Statement. Informed consent was obtained from all subjects and the study protocol was approved by the hospital ethics committee.
A total of 115 consecutive patients with DM (age range 18-86 years) participated in the study. Sample size was determined through power analysis. Recruitment started on 1 October 2012. The follow-up was terminated on 30 July 2014. Patients were randomly assigned (2:1), using random number generator and sealed envelopes, into two groups matched for age, BMI and HbA1c. Group 1 consisted of 70 DM patients who participated in the telemonitoring (telemonitoring group-TG). Group 2 included 35 DM patients under bimonthly outpatient care at the Department of Endocrinology (control group-CG) (Table 1) (Figure 1). Patients of all groups attended an initial 2-hour educational session for DM self-management, recognition and management of hypoglycemia and nutrition. TG participants were informed of their intervention assignments and instructed on the use of the device, performed one supervised test transmission of glucose data and completed a questionnaire regarding their net daily salary and the total financial cost of a potential regular outpatient department visit. Patients in both groups were further divided into subgroups depending on their baseline HbA1c levels. Recently diagnosed and hospitalized DM patients with baseline HbA1c ≥10% (86 mmol/mol) were assigned to groups TG10 and CG10. Groups TG7.5 and CG7.5 included insulin-treated DM patients with insufficient control [(7.5%≤HbA1c<10%) (58≤HbA1c<86 mmol/mol)] despite at least two years of follow-up at the outpatient department. TG and CG patients were also divided into type 1 (TG1 and CG1) and type 2 DM subgroups (TG2 and CG2) (Table 2).
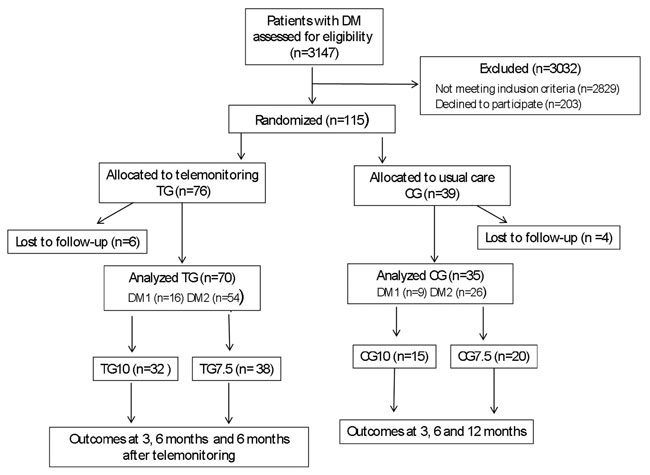
Figure 1. Flow diagram of the study: TG, telemonitoring group; CG, control group; TG10, telemonitoring group with initial HbA1c ≥10%; CG10, control group with initial HbA1c≥10%; TG7.5, telemonitoring group with 7.5%≤HbA1c<10%; CG7.5, control group with 7.5%≤HbA1c<10%; DM1, diabetes mellitus type 1; DM2 diabetes mellitus type 2.
Intervention
CG patients received bimonthly care as provided by outpatient department endocrinologists. TG patients received a 6-month DM management active-support intervention. An 8x5x3 cm USB-connected modem, compatible with diverse brand name glucose-meters, was provided. Data were transmitted to the computers of the Department of Endocrinology via this modem. Patients in both groups were encouraged to perform at least 4 measurements per day, while TG patients were instructed to transmit data at least once per day. Monitoring was combined with a coordinated management and feedback system based on transmitted data. Every time that the patient connected the modem to the glucose-meter, all previous glucose measurements were transmitted. Storage, documentation and communication with patients were achieved through specialized integrated software (Τelemedicor, Hertfordshire-UK). An endocrinologist reviewed the data and contacted participants via mobile phone SMS or e-mails when necessary. Frequency of doctor to patient communication varied among TG patients depending on glucose values and compliance (mean 1 time/week/patient). Alerts were also set for high [>300 mg/dL (16.7 mmol/lt)] or low [<70 mg/dL (3.9 mmol/lt)] glucose levels. When alerts were activated, the endocrinologist received a mobile phone text-message thus allowing prompt communication and proper therapeutic adjustments. If desired by the patient, alerts were also sent to family members. Frequency of measurements for each TG patient was directly provided from the telemonitoring software, whereas frequency of measurements in the CG was calculated through recorded data on the patients’ glucose-meter memory.
Measurement of HbA1c and evaluation of BMI were performed at enrolment and at 3 and 6 months on telemonitoring as well as 6 months after discontinuation of the telemonitoring in all groups. HbA1c was measured by HPLC (MENARINI diagnostics HA-8160, within-run CVs for medium and high value samples: 0.6% and 0.7%, respectively).
Statistical analysis
Statistical analysis was performed using the SPSS software package (SPSS Inc., version 17.0, Chicago, USA). Within-group comparison was performed with one-way ANOVA and t-test in normally distributed values, whereas one-way ANOVA plus the Wilcoxon test were utilized in non-normally distributed values. Between-group comparisons were performed by using a t-test in the case of normally distributed values and Mann-Whitney in non-normally distributed ones. Primary outcome was considered an improvement in HbA1c level. Secondary outcomes included changes in BMI, frequency of hyper-/hypoglycemias and cost. Associations between parameters were estimated in both groups with the use of Pearson or Spearman correlation coefficients. To further investigate the association between ΗbA1c between the two groups over time we also fitted a repeated measures ANOVA to assess basic group effect, and time as a structural effect, while additionally allowing for possible group-time interaction. Moreover, we also tested gender, age and BMI as potential confounders. P value <0.05 was considered to be statistically significant.
RESULTS
HbA1c and BMI
There were no significant differences regarding age, sex, BMI and number of glucose measurements per day between TG and CG at baseline. Within-group analysis revealed a statistically significant reduction in HbA1c levels in all TG subgroups at 3 and 6 months of telemonitoring as well as 6 months after its discontinuation, compared to enrolment (Table 1). A significant BMI reduction was observed in the TG at 6 months on telemonitoring as well as at 6 months off telemonitoring compared to baseline. Subgroup analysis revealed that this reduction regarded the TG7.5 group, while no difference was observed in TG10.
Between-group comparisons revealed a significant reduction in HbA1c at 3 and 6 months on telemonitoring as well as at 6 months after its discontinuation in all TG compared to CG groups (Figure 2, Figure 3). However, between-group analysis found no significant BMI alterations in TG compared to CG in any of the subgroups compared.

Figure 2. Combined boxplot diagrams of mean values ±SD of HbA1c and scatter diagrams over time in different DM subgroups. A. All patients and controls with type 1 and 2 DM. B. Patients and controls with type 1 and 2 DM (HbA1c≥10%). C. Patients and controls with type 1 and 2 DM (7.5≤HbA1c<10%). D. Patients and controls with type 2 DM.
CG: control group; TG: telemonitoring group; TM: telemonitoring; DM: diabetes mellitus.

Figure 3. Mean values ±SΕ of HbA1c and BMI over time in different DM subgroups. A. All patients and controls with type 1 and 2 DM. B. Patients and controls with type 1 and 2 DM (HbA1c≥10%). C. Patients and controls with type 1 and 2 DM (7.5≤HbA1c<10%).
CG: control group; TG: telemonitoring group; TM: telemonitoring; DM: diabetes mellitus.
Regarding DM type, there were no significant differences as to age, sex, BMI and glucose measurements per day between TG1 and CG1 as well as TG2 and CG2 subgroups. Within-group analysis revealed a statistically significant reduction in HbA1c of both TG1 and TG2 patients at 3 and 6 months of telemonitoring as well 6 months following its discontinuation, compared to the baseline HbA1c levels (Table 2). Between-group analysis showed a significant reduction in HbA1c at 3 and 6 months on telemonitoring as well as at 6 months after its discontinuation in TG2 in comparison to CG2 subgroup (Figure 3). On the other hand, even though HbA1c reduction in TG1 was significant at 3 months of telemonitoring compared to CG1, it did not reach a statistically significant difference at 6 months of telemonitoring or at 6 months after its discontinuation compared to CG1 (Table 2).
The repeated measures ANOVA analysis showed that group (p=0.003), time (p<0.001) and their interaction (p=0.008) had statistically significant effects. Moreover, none of the potential confounders we assesed (i.e. gender, age and BMI) had statistically significant effects. Therefore, we conclude that the levels of ΗbA1c differ between the two groups and this difference is not constant over time.
No significant change was documented between TG1, TG2 and CG1, CG2 subgroups regarding patients’ BMIs (Table 2).
Frequency of glucose measurements and doctor-to-patient communication
TG patients performed 3.43±1.06 (mean±SD) blood glucose measurements per day and CG patients 3.44±1.12 during the 6-month intervention period (NS). Blood glucose measurements per day in TG10, CG10 and in TG7.5, CG7.5 are presented in Table 2. During the 6 months following discontinuation of telemonitoring, the number of glucose measurements per day did not differ significantly in TG and CG patients (data not shown). No significant correlation was detected between frequency of glucose measurements and HbA1c or BMI in any of the TG and CG analysed.
Mean frequency of doctor-to-patient communication varied among TG patients depending on glucose values and compliance (mean: 1 time/week). Mean time dedicated per TG patient per month was estimated at an average of 10 min based on data extracted by the software used.
Frequency of hypo- and hyperglycemias
Number of both mild [50<Glc≤70mg/dL (2.8<Glc≤3.9mmol/lt)] and severe [≤50mg/dL (≤2.8mmol/lt)] hypoglycemias per month were reduced in the TG compared to the CG over the 6-month period of telemonitoring. The median number of mild hypoglycemias in TG and CG during the 6 months of the telemonitoring was: 0 (min=0, max=17) vs 3 (min=0, max=17), respectively (p<0.0001) [first month of telemonitoring: 3.5 (min=0, max=30) vs 5 (min=0, max=23), NS], and of severe hypoglycemias hypoglycemias in TG and CG: 0 (min=0, max=7) vs 2 (min=0, max=12), respectively (p<0.0001) [first month of telemonitoring: 2 (min= 0, max=25) vs 3 (min=0, max=17), NS].
Furthermore, the median number of profound hyperglycemias [≥300mg/dL (≥16.7mmol/lt)] was also reduced in the TG compared to the CG patients during the 6 months of the telemonitoring period [0 (min=0, max=11) vs 3 (min=0, max=8), respectively (p<0.0001)] [first month of telemonitoring: 2 (min=0, max=26) vs 3 (min=0, max=33), respectively, NS].
Cost
The cost of telemonitoring for each patient was estimated at 50 Euros bimonthly based on data provided by the modem and software provider (Telemedicor, Hertfordshire-UK). The estimated total cost of conventional outpatient department follow-up for TG was evaluated by adding transportation cost, standard 5 Euros hospital fee and other expenses based on patients’ structured questionnaires answers. Transportation cost depended on the means of transportation used by each patient and any accompanying persons (ship, airplane, train or bus tickets, taxi fare or estimated oil/petrol expenses in the case of private car use). Other expenses included money spent on stay, food, beverages and additional expenses during travel to the hospital. If TG patients had visited the outpatient department, mean cost estimates would have been 39.21±76.93 (median: 12, minimum: 5, maximum: 400) euros per each bimonthly visit. However, 22 TG patients would have paid more than 50 euros bimonthly if they had been followed at the outpatient department. All of these patients were either island inhabitants or their residence was located at more than 100 km from the hospital facilities.
Forty-four out of 70 TG patients were either pensioners (n=32) or unemployed (n=12). The value of net daily salary for the remaining 26 employed patients was calculated at 67.2±49.69 euros/day.
DISCUSSION
During the coming years, fundamental changes in DM care may be required in order to overcome cost pressures on health care providers. New approaches, such as telemonitoring, could play crucial role in early detection of DM dysregulation, improvement of HbA1c and prevention of DM complications.
In the present study, we evaluated the efficiency of telemonitoring and active intervention in suboptimally insulin-treated DM patients as well as in newly-diagnosed DM patients with HbA1c ≥10% in terms of HbA1c and BMI improvement. The cost of telemonitoring has also been studied, thus addressing a question that has received little attention in the literature. Duration of any potential beneficial effects was also studied 6 months after its discontinuation.
In contrast to previous trials that did not include active intervention in response to real-time blood-glucose transmission, the present study involved active medication management. Only a limited number of trials have reported medication adjustments within 24 to 72h.23,31 In our study, therapeutic adjustment in response to alerts was offered within 1 hour during the day and 7 hours during the night (maximum).
Between-group analysis showed that patients in the TG had a greater HbA1c improvement compared to the CG. This was also observed for TG10 patients compared to CG10 and for TG7.5 patients compared to CG7.5. Of note, HbA1c improvement was detected as early as 3 months after initiating telemonitoring in all TG groups. In the study of Stone et al, a HbA1c reduction of 1.7 vs 0.7% was observed at 3 months and 1.7 vs 0.8% at 6 months in the TG compared to the CG, respectively; however, the CG received a monthly coordinating phone call.23
Further assignment of TG and CG participants into subgroups depending on baseline HbA1c enabled us to determine that patients with initial HbA1c≥10% were those who most benefited from telemonitoring. This has also been observed in previous studies which demonstrated a tendency of greater HbA1c reduction in the TG with higher baseline HbA1c.32 This could partially be interpreted by the greater capability to lower HbA1c in the TG10 patients, since mean HbA1c at enrolment was greater in that patient group. Greater intensification of insulin therapy in the TG may have contributed significantly to the marked reduction in HbA1c in the TG in comparison to CG.
Further analysis revealed that the greater improvement of HbA1c in type 1 compared with type 2 DM patients did not reach statistical significance at 6 months on telemonitoring or at 6 months following its discontinuation. This could be attributed to the small number of type 1 DM in our study. The number of participants needed to achieve study objectives were determined by power analysis (data at http://www.anzctr.org.au/TrialSearch.aspx, ACTRN12614000977673). We found that for a significance level of 0.05, our study should recruit 23 subjects per group to achieve a 90% power or 17 subjects per group in order to obtain an 80% power. Unfortunately, time restrictions prevented us from enrolling more type 1 DM patients. Even though the between-group (TG1 vs CG1) analysis is not sufficiently powered, it demonstrates that a similar trend of HbA1c reduction exists in DM1 patients during the telemonitoring period. In the meta-analysis of Marcolino et al, a more pronounced reduction of HbA1c was observed in type 1 DM patients that could be attributed to the fact that such patients are younger, more keen and more motivated towards glycemic control.32
Only two RCTs have previously assessed the effect of telemonitoring on patients’ BMI and found a non-significant reduction.20,21 Our study came up with similar results. Within-group and subgroup analysis showed that the only significant BMI reduction regarded the TG7.5. This reduction also had a “carry-over” effect in the TG group. This finding could be justified by the fact that TG7.5 included less dysregulated DM patients who might be more motivated and determined to accomplish an overall change in their daily habits regarding diet and exercise. In addition, minimizing large variations in blood glucose levels and optimizing insulin distribution during the day, as reflected by a decreased hyper- and hypoglycemias rate, can result in reducing the daily insulin dosage thus eliminating weight gain that is associated with it. Of note, between-group analysis found no significant BMI alterations in TG compared to CG in any of the subgroups compared.
It is uncertain whether the improved glycemic control observed in the TG could be sustained beyond 6 months without telemonitoring. Only one study has previously addressed the duration of telemonitoring effects. However, the CG in that trial received phone calls as a lower intensity intervention instead of the usual outpatient department care.33 Extension of patients’ follow-up showed that HbA1c improvement achieved by the end of the telemonitoring was only slightly attenuated 6 months following its discontinuation. This could be attributed to the increased motivation and compliance or potential carry-over effects from experience in frequent medication adjustment acquired by TG patients in the initial 6 months of telemonitoring.
It is noteworthy that the baseline number of daily blood glucose measurements did not differ among the TG and CG, while no correlation was established between number of blood glucose measurements and HbA1c during the 6 months of telemonitoring and the 6-month period following its discontinuation. The relation between frequency of glucose measurements and glycemic control is complex and inconsistent. Observations from the National Health and Nutrition Examination Survey (NHANES) revealed that only 29% of DM patients performed blood glucose measurements at least daily.34 In the study of Stone et al, a mean of 2.3 measurements per day was achieved by TG patients, while data on CG were not available. However, no significant association was detected between frequency of glucose measurements and magnitude of decline in HbA1c within the TG.23,34
Increased contact time between patients and health care specialists has been associated with lower HbA1c levels.35 Previous studies report that telemonitoring interventions may require more provider time than does traditional diabetes management.17,29 However, results from our study indicate that low-intensity contact could be effective in maintaining improvements in glycemic control over a 6-month period.
HbA1c as an endpoint has certain limitations since it may be normal in patients who alternate between hyperglycemia and hypoglycemia. An advantage of our study is that we further assessed the frequency of hypo- and hyperglycemia. Telemonitoring resulted in the decrease of both hypo- and hyperglycemia in all TG subgroups. More importantly, the number of cases of severe hypoglycemia was significantly reduced in the TG. This is of utmost importance, since there have been concerns that tight glycemic control may not be appropriate for all patients, especially those of older age with comorbidities. The ACCORD trial reported increased mortality in a subgroup of patients on intensive glycemic control, whereas the UKPDS and VA Diabetes Trial reported decreased cardiovascular events in younger patients and in cases where tight glycemic control had been started early in the course of type 2 DM.36-38
The economic viability of implementing new DM management approaches is of great importance and should be estimated through long-term RCTs and analysis of all parameters for the respective intervention used. Whether telemonitoring DM patients with inadequate glycemic control is a cost-effective approach is not easy to estimate. Costs for the patient as well as costs for the health provider are composed of diverse parameters which should be evaluated. All these parameters may vary from one country to another or even from one region to another. Regarding cost for the patient, one should take into consideration transportation costs, hospital fee and other expenses. The loss of working hours for patients who frequently visit the outpatient department or are hospitalized should also be evaluated. For patients living far from specialized DM centers (remote islands or mountainous regions), cost-effectiveness due to avoidance of transportation expenses is clear. Several studies on the economic costs and benefits of such interventions suggest that patients benefit from telemedicine in terms of the reduced time and travel costs relative to routine care.17,34 However, data regarding the cost-effectiveness are scarce and controversial since cost is not uniformly measured.32 In our study, cost of telemonitoring service (software, modem, internet/mobile communication cost and provider expenses) was estimated at 25 Euros/month. We observed that in the case of patients living within a 100 km range of the mainland health care facility, telemonitoring offered no financial benefit to the patients. However, contract data supplied by the telemoninoring provider revealed that charges could be substantially reduced depending on the number of patients participating in the telemonitoring (>250 patients/year: 20 Euros/month, >500 patients/year: 15 Euros/month, >1000 patients/year: 10 Euros/month). Furthermore, if such an intervention were to be covered by health providers, the cost of its use could be substantially reduced. The cost of TM for the national health provider is far more complicated to calculate. Data to be taken into consideration includ the salaries of the personnel of the public health system involved in this task divided by the time spent per patient during morning and afternoon hours while the physician is on shift duty (10 minutes per month per patient plus the initial 2-hour DM educational session, which was organized 20 times). Therapeutic adjustments were not provided during night hours and thus could not be cost-evaluated. For such a cost to be calculated, one should take into consideration a potential salary for non-medical personnel who would assist with any urgent adjustments needed during night hours. Data presented in this study apply in the case of Greece and the specific telemonitoring provider, whereas different charges and expenses will apply in other countries.
By design, our study focused on patients with DM and suboptimal glucose control. Thus, data regarding changes in blood pressure or cholesterol levels were not collected. This prevented us from assessing any potential combined beneficial effects of improved DM control on these parameters. Data from a recent meta-analysis showed no clinically relevant impact of DM telemonitoring on LDL or blood pressure.32 In addition, even though participants in both CG and TG were demographically similar, we cannot exclude that those in the TG might be more motivated to improve their glycemic control than the typical DM patient. Unfortunately, it was not feasible to link cases of hyper- and hypoglycemia with specific timepoints regarding food consumption, since the software does not currently support data-transmission on food intake along with pre- and postprandial data on glucose levels. Another limitation of our study is that the individuals studied were predominantly males (64%) compared to the meta-analysis of previous studies (56% males). However, no significant sex-related differences were found in terms of HbA1c and BMI reduction in any of the groups in any of the telemonitoring phases or following its discontinuation. Moreover, telemonitoring resulted in short-term improvements in HbA1c. Whether a long-term improvement has a beneficial effect on cardiovascular risk, as well as the magnitude of such an effect, needs studies of longer duration that would include cardiovascular risk assessment.
In conclusion, telemonitoring has been proven effective in rapidly reducing HbA1c, with the greatest reductions obtained by 3 months, sustained at 6 months and slightly attenuated at 6 months after its discontinuation. This improvement could be attributed to better compliance due to a more frequent doctor-patient contact and to greater intensification of insulin therapy in the TG. Moreover, frequency of hypo- and hyperglycemia cases was also reduced. Finally, a reduction of visits to outpatient departments was achieved, resulting in lower cost for certain patient groups and less patient inconvenience. In the near future, telemonitoring could help prevent acute hospitalizations due to DM complications while offering an alternative to conventional outpatient department visits, thus maintaining individuals in their own community.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Spyros Koutmos, Dr. Theodora Kounadi, Dr. Chrisanthi Marakaki and Dr. Dimosthenis Malliopoulos for their assistance.
REFERENCES
1. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE, 2014 Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103: 137-149.
2. Patterson C, Guariguata L, Dahlquist G, Soltész G, Ogle G, Silink M, 2014 Diabetes in the young - a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract 103: 161-175.
3. Whiting DR, Guariguata L, Weil C, Shaw J, 2011 IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94: 311-321.
4. Beagley J, Guariguata L, Weil C, Motala AA, 2014 Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract 103: 150-160.
5. Stratton IM, Adler AI, Neil HAW, et al, 2000 Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321: 405-412.
6. UKPDS Group, 1998 Effect of intensive blood glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352: 854-865.
7. Diabetes Control and Complications Trial Research Group, 1993 The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977-987.
8. UK Prospective Diabetes Study (UKPDS) Group, 1998 Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837-853.
9. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA, 2008 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577-1589.
10. Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group, 2005 Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353: 2643-2653.
11. Stahl-Pehe A, Lange K, Bächle C, Castillo K, Holl RW, Rosenbauer J, 2014 Mental health problems among adolescents with early-onset and long-duration type 1 diabetes and their association with quality of life: a population-based survey. PLoS One 9: e92473.
12. Hood KK, Rausch JR, Dolan LM, 2011 Depressive symptoms predict change in glycemic control in adolescents with type 1 diabetes: rates, magnitude, and moderators of change. Pediatr Diabetes 12: 718-723.
13. Ralston JD, Hirsch IB, Hoath J, Mullen M, Cheadle A, Goldberg HI, 2009 Web-based collaborative care for type 2 diabetes: a pilot randomized trial. Diabetes Care 32: 234-239.
14. Yoon KH, Kim HS, 2008 A short message service by cellular phone in type 2 diabetic patients for 12 months. Diabetes Res Clin Pract 79: 256-261.
15. Kim SI, Kim HS, 2008 Effectiveness of mobile and internet intervention in patients with obese type 2 diabetes. Int J Med Inform 77: 399-404.
16. Harno K, Kauppinen-Makelin R, Syrjalainen J, 2006 Managing diabetes care using an integrated regional e-health approach. J Telemed Telecare 12: Suppl 1: 13-15.
17. Shea S, Weinstock RS, Starren J, et al, 2006 A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc 13: 40-51.
18. McMahon GT, Gomes HE, Hickson HS, Hu TM, Levine BA, Conlin PR, 2005 Web-based care management in patients with poorly controlled diabetes. Diabetes Care 28: 1624-1629.
19. Wakefield BJ, Holman JE, Ray A, et al, 2011 Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemed J E Health 17: 254-261.
20. Bujnowska-Fedak MM, Puchała E, Steciwko A, 2011 The impact of telehome care on health status and quality of life among patients with diabetes in a primary care setting in Poland. Telemed J E Health 17: 153-163.
21. Rodríguez-Idígoras MI, Sepúlveda-Muñoz J, Sanchez-Garrido-Escudero R, et al, 2009 Telemedicine influence on the follow-up of type 2 diabetes patients. Diabetes Technol Ther 11: 431-437.
22. Izquierdo R, Lagua CT, Meyer S, et al, 2010 Telemedicine intervention effects on waist circumference and body mass index in the IDEATel project. Diabetes Technol Ther 12: 213-220.
23. Stone RA, Rao RH, Sevick MA, et al, 2010 Active care management supported by home telemonitoring in veterans with type 2 diabetes: the DiaTel randomized controlled trial. Diabetes Care 33: 478-484.
24. Bond GE, Burr R, Wolf FM, et al, 2007 The effects of a web-based intervention on the physical outcomes associated with diabetes among adults age 60 and older: a randomized trial. Diabetes Technol Ther 9: 52-59.
25. Piette JD, Weinberger M, McPhee SJ, et al, 2000 Do automated calls with nurse follow-up improve self-care and glycemic control among vulnerable patients with diabetes? Am J Med 108: 20-27.
26. Piette JD, Weinberger M, Kraemer FB, McPhee SJ, 2001 Impact of automated calls with nurse follow-up on diabetes treatment outcomes in a Department of Veterans Affairs Health Care System: a randomized controlled trial. Diabetes Care 24: 202-208.
27. Montori VM, Helgemoe PK, Guyatt GH, et al, 2004 Telecare for patients with type 1 diabetes and inadequate glycemic control: a randomized controlled trial and meta-analysis. Diabetes Care 27: 1088-1094.
28. McManus RJ, Mant J, Bray EP, et al, 2010 Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomized controlled trial. Lancet 376: 163-172.
29. Biermann E, Dietrich W, Rihl J, Standl E, 2002 Are there time and cost savings by using telemanagement for patients on intensified insulin therapy? A randomized, controlled trial. Comput Methods Programs Biomed 69: 137-146.
30. Chase HP, Pearson JA, Wightman C, Roberts MD, Oderberg AD, Garg SK, 2003 Modem transmission of glucose valuesreduces the costs and need for clinic visits. Diabetes Care 26: 1475-1479.
31. Cho JH, Song KH, Chang SA, et al, 2006 Long-term effect of the Internet-based glucose monitoring system on HbA1c reduction and glucose stability. Diabetes Care 29: 2625e31.
32. Marcolino MS, Maia JX, Alkmim MBM, Boersma E, Ribeiro AL, 2013 Telemedicine Application in the Care of Diabetes Patients: Systematic Review and Meta-Analysis. Plos One 8: e79246.
33. Stone RA, Sevick MA, Rao RH, et al, 2012 The Diabetes Telemonitoring Study Extension: an exploratory randomized comparison of alternative interventions to maintain glycemic control after withdrawal of diabetes home telemonitoring. J Am Med Inform Assoc 19: 973-979.
34. Sarol JN Jr, Nicodemus NA Jr, Tan KM, Grava MB, 2005 Self-monitoring of blood glucose as part of a multicomponent therapy among non-insulin requiring type 2 diabetes patients: a meta-analysis (1966– 2004). Curr Med Res Opin 21: 173-184.
35. Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM, 2002 Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care 25: 1159-1171.
36. Action to Control Cardiovascular Risk in Diabetes Study Group, 2008 Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358: 2545-2559.
37. Duckworth W, Abraira C, Moritz T, et al, 2009 Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129-139.
38. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA, 2008 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577-1589.
Address for correspondence:
Stelios Fountoulakis, Department
of Endocrinology and Diabetes Center, Athens General Hospital “G. Gennimatas”,
154 Mesogion Avenue 11527 Athens, Greece, Tel: +30 2107768283, Fax: +30
2107779146, E-mail: st_foun@yahoo.com
Received: 09-01-15, Accepted: 17-06-15