Professor Emeritus of Endocrinology, Athens University Medical School, Athens, Greece
Androgenetic alopecia, Androgens, Baldness, Cardiovascular disease, Estrogens, Female Pattern Hair Loss, Prostate cancer, Testosterone
THE ENDOCRINOLOGY OF BALDNESS
Male pattern baldness, scientifically known as androgenetic alopecia (AGA), is a very common condition, universally prevalent, which progresses with aging. The loss of scalp hair of any degree both in men and women is a cause of considerable psychological distress associated with a loss of self-esteem and feelings of diminished self-image, resulting in introversion and depression. Particularly among men, the very manifest obviousness of baldness is often a source of major psychological anguish.
Two clinical features of male baldness have been recognized: a predisposition to scalp hair loss inherited from the father or grandfather(s) and a negative connection of scalp hair loss with the testes. Eunuchs and pre-pubertally castrated men who fail to develop body hair have normal scalp hair which shows no sign of loss with age. However, castrates receiving lifelong replacement therapy with testosterone show scalp hair loss and various degrees of baldness. The complete restoration of body hair in eunuchs and castrates by the administration of testosterone established the notion that androgens are responsible for hair growth, but at the same time it revealed the hair loss effect of testosterone on scalp hair.1 The paradox of a diametrically different action of a hormone on its receptors aroused much interest and initiated research into the dual effect of testosterone and into the investigation of this biological aberrance that has created an unwanted phenotypic manifestation which, because of its great prevalence, the psychological impact and the difficulties and cost of treatment, is an important medicosocial problem.
This review is a discussion on the endocrine connections at the hormonal, molecular and genetic levels with male and female scalp hair loss and on the psychological effects. Also discussed is the theory that baldness may be an early prognostic marker of prostate cancer and cardiovascular disease.
THE PSYCHOLOGICAL EFFECTS OF BALDNESS
Head hair is an essential complimentary feature to the face in the composition of an individual’s physiognomy and, by virtue of its natural qualities and its capacity to be modeled artistically, contributes significantly to an individual’s self-perceived image as well as to the response of others. It is therefore only natural that hair loss to any degree will have a negative psychological impact on the quality of a person’s life. Several studies have documented the psychological distress caused by baldness and investigated its parameters.
With regard to males, the psychological impact that balding men attribute to hair loss was investigated in 63 men with modest balding, 40 men with more extensive balding and 42 non-balding controls.2 Effects of balding reflected considerable anxiety, moderate stress or distress and compensatory efforts that were especially common among younger men. Compared to controls, balding men had less body-image satisfaction. Similar psychological effects were observed in 182 men with a wide range of ages and hair loss varying from none to severe.3 An increasing degree of hair loss was associated with loss of self-esteem, depression, introversion, neuroticism and the feeling of being unattractive. Loss of self-esteem, introversion and feeling unattractive particularly affected young men. The perception of men with baldness by women and non-balding men was studied in Korea via a questionnaire carried out among 130 women 15-58 years old, 90 non-balding men 18-72 years old and 30 balding men 20-63 years old.4 Balding men were perceived as being older and less attractive by over 90% of responders. Less than half of the responders perceived balding men as less confident, duller or less potent. Significantly more women than non-balding men perceived balding men as less attractive.
Via randomly selected samples of 1,717 men 18 to 40 years of age and a questionnaire comprised of 78 questions specific to hair loss, a survey was conducted in four European countries, France (n=502), Germany (n=508), Italy (n=383) and the United Kingdom (n=324), with such meticulousness that the questionnaire which was originally developed in the States was translated into British-English to avoid misunderstandings.5 Men were categorized into one of four groups based on their self-reported degree of hair loss as “a full head of hair”, “a little hair loss”, “some hair loss” or “moderate hair loss” and “considerable hair loss”, “a lot of hair loss” or “bald”. Of men under 20 years of age, 81.3% reported that they had “a full head of hair”, whereas only 35.2% of men 35 years and older reported “a full head of hair”, with about 30% reporting at least “moderate hair loss”. Hair loss distress increased with increasing hair loss in all four countries. For Germany the distress scores were greater in young men. Hair loss distress in all countries concerned mainly the worry inflicted by hair loss itself rather than looking older. Overall, 98% of men with a “full head of hair” reported that hair loss was not at all merely slightly noticeable, whereas 75% of men reporting “I am bald” stated their perception that their hair loss was very or extremely noticeable by others.
THE INCIDENCE OF BALDNESS
The very widespread incidence of baldness either as thinning of hair or denudation areas on the scalp in aging men is obvious. Ethnic differences in the appearance of baldness have also been traditionally recognized. However, large-scale population studies of the characteristics and racial differences in this interesting and very common human phenotypic trait are limited. Hamilton in 1951, summarizing his pioneering clinical studies in baldness, established eight types of scalp hairiness that could be used as standards for the classification and the grading of the extent of baldness and categorized 312 Caucasian males, 214 Caucasian females and 77 Chinese males aged 20-89 years according to his classification.6 He noticed a progressive increase of male baldness with age, reaching a peak of 85% incidence in their 7th decade in 32 males, whereas in females the highest incidence was 30%. The 77 Chinese also had a lower incidence but with a similar increase with advancing age (data from Figure 5). The author also sought to investigate the endocrine profile of the men with baldness employing the now outdated methods of the time. He reported that the androgen metabolites in urinary 17-ketosteroids were similar in men regardless of whether or not they were bald.
Based on the examination of a large cohort of 1,000 men, Norwood in 1975 slightly modified Hamilton’s classification of AGA pattern types establishing seven types of balding that have ever since served as a reference source in dermatological practice and investigation.7 Type I is a scalp with no or minimal recession of the frontopariental hair line. Type II is a scalp with frontoparietal recession. Type III are scalps with deep frontoparietal recession and types IV to VII are scalps with severe frontoparietal recession and various degrees of absence of hair on the vertex, this latter type being the most severe degree of frontoparietal recession in which all that remains is a narrow band of hair beginning laterally just anterior to the ears and extending posteriorly on the sides and quite low on the occipital area (Figure 1). The incidence of this last type was 4-5% in 321 men aged 40-59 years, 10-11% in 242 men aged 60-79 years and 17% in 77 men 80 years old and over.

Figure 1. The Hamilton-Norwood classification of male pattern of baldness types. Type I scalp with no or minimal recession of frontoparietal hair line, Type II and III scalps with moderate and deep recession, Types IV to VII scalps with severe recession and various degrees of absence of hair on the vertex, Type VII being the most severe recession in which all that remains is a narrow band of hair beginning laterally anterior to the ears and extending posteriously low on the occiput.
Direct observations of AGA were made in a population-based study of prostate cancer in 1,390 men aged 40-69 years in Australia who were grouped into four categories: a) no AGA, b) frontal AGA, c) vertex AGA and d) full AGA.8 Applying this simpler classification, it was found that the prevalence of vertex AGA and full AGA increased with age from 31% in the age group 40-55 years to 53% in the age group 65-69 years. Only the frontal type was similar in both age groups 31-32%. No association between AGA and smoking or benign prostatic hypertrophy was identified.
A high frequency of AGA in men less than 50 years old was observed in a study of self-reported degree of hair loss rated by a trained reporter in a community-based sample of healthy men aged 18-49 years.9 The proportion of men with moderate to extreme hair loss (Type III or greater) was 42%, increasing with age from 16% for men 18-29 years old to 53% of men 40-49 years old. A high incidence and severity of AGA was also self-reported by 4,101 men of a Norwegian community.10 One in five (19.5%) of men aged less than 50 years reported baldness of Type V or more of the Hamilton Norwood classification (for Types V-VII, see Figure 1).
A high incidence of balding in men was also reported in Sheffield, Britain, by Birch and Messenger in 572 men of ages distributed evenly between 16 and 91 years.11 It was determined that, by 70 years of age, 80% of men had balding and half of them had severe balding (grades VI and VII on the Hamilton/Norwood scale). According to the authors, all these men will eventually go bald, given that the frequency rate of balding was increasing. Almost all men who developed balding before the age of 30 years had a balding father, indicating a strong genetic association, making bald men of this age group appropriate candidates for genetic analysis.
The prevalence of male and female pattern hair loss was studied in 1,450 men and women who responded to a postal survey of 5,000 men and women living in Maryborough, a small town of 8,000 people in the state of Victoria, Australia.12 Ninety-eight percent of men aged 80 years and over had bitemporal recession of hair and 73.5% mid-frontal hair loss. The corresponding prevalence in women was 64.4% and 57%.
The difference in prevalence of AGA in Asiatic populations observed by Hamilton has been confirmed by studies in populations in Singapore, Thailand, Korea and China. A questionnaire-based survey in the district of Bishan, Singapore, showed an incidence of 63% of AGA in 254 men according to the Norwood criteria.13 The prevalence increased from 32% among adults aged 17-26 years to 100% among those in their 80s. Proportionately more Indians (87%) were affected compared to Chinese (61%). The authors made the interesting observation that 81% of men with AGA did not seek help as they did not view their condition as a problem.
The prevalence of baldness in the population of Korea was found to be significantly low as compared to Caucasians.14 In a study of 5,531 men and 4,601 women in Seoul, the prevalence of Type III or above AGA in men was 14.1% at all ages and increased steadily with advancing age, being lower than that of Caucasians of all age groups: 2.3% in the 3rd decade, 4% in the 4th, 10.8% in the 5th, 24.5% in the 6th, 34.3% in the 7th decade and 49.9% in men over 70 years old. A family history of baldness was present in 48.8% of men.
Two fairly recent (2009 and 2010) Chinese large population-based studies have documented the lower incidence of baldness in Chinese, which is similar to that of the Koreans. In a population-based study of 3,519 men in a community in Shanghai, the overall prevalence of AGA was 19.9% advancing with age.15 Very low incidence was noted in men less than 50 years old. In the 1st to the 3rd decade, the prevalence was 0.3-0.4%, in the 4th decade 2.7%, in the 5th decade 10.1%, followed by 20% in the 6th, 43.5% in the 7th decade and 60% in men over 70 years old. A positive family history of AGA existed in 55.8% of the men. Similar results were obtained in a community-based study of 6,811 men in six Chinese cities.16 The prevalence of AGA was 21.3%, increasing with age. In men 18-29 years old the prevalence was 2.8% in the 4th decade, 13.3%, 21.4% in the 5th, 31.9% in the 6th, 36.2% in the 7th decade and 41.4% in those aged 70 years and over (Figure 2). Earlier, in 2002, Pathomvanich et al based on their own investigations questioned the common belief that Asians had a lower incidence of baldness than Caucasians.17 In a total of 1,124 Thai and Chinese men residents in Bangkok Thailand, the prevalence of AGA and the increase with age approached that of Caucasians. The authors suggest that the socioeconomic environment and the westernized diet may be contributing to this high prevalence. The characteristics, the population differences, the prevalence and the types of AGA in Asiatic men and women are discussed in a review by Lee and Lee.18

Figure 2. Incidence of baldness in males at various decades in Caucasian (solid line) and Asiatic (broken line) large population series (1. Hamilton,6 2. Norwood,7 3. Severi,8 4. Gan,12 5. Birch,11 6. Paik,14 7. Wang,16 8. Xu15).
THE HAIR IN ANDROGENETIC ALOPECIA
The hair is the part of the organism possessing the most vigorous metabolic and proliferative activity stimulated by the androgens. No other organ in males, with the exception of spermatogenesis, shows such continuous and cyclic development and regeneration. The normal growth cycle of hair proceeds in three phases. First, there is a long period of development which in the scalp may last for two years or more: this is the anagen phase during which hair growth begins by epithelial cell division of an enlargement as its base, the bulb. The hair bulb surrounds an extension of the epidermis, the dermal papilla, which is a true laboratory rich in androgen receptors and receptor transcriptional coregulators, active in androgen synthesis and metabolism as well as growth factors and cytokine production that induce the hair bulb activity. The termination of the anagen phase is produced by cessation of cell division that is followed by a phase of regression, the catagen. The length of the hair shortens by regression of its lower part and the hair becomes thinner and miniaturized. The dermal patella also regresses showing reduction and inactivation of its cells. The last phase of the hair growth cycle, the telogen, is a short period of variable time ranging from 1 to 3 months. At the end of telogen the dermal patella is reactivated, cellar division commences and a long new phase of anagen begins forming a new hair.19-21
In androgenetic alopecia, there is a dramatic shortening of the hair cycle at the expense of the anagen phase. The duration of this period of hair development, which varies in the different parts of the body and in the scalp lasts for many months, is reduced to a few weeks or months in the areas of baldness. The telogen phase remains the same or is moderately lengthened, resulting in an accumulation of miniaturized anagen hair at this phase in the scalp that is easily shed through combing and washing (Figure 3).

Figure 3. Schematic representation of scalp hair cycle. Normally, the development period, the anagen phase (1-5), lasts for many months followed by a phase of regression, the catagen (5-8), during which the hair becomes shorter, thinner and miniaturized. The last phase, the telogen, is a short period of 1 to 3 months of rest followed by the regeneration of hair and the beginning of a new cycle of development. In baldness, the hair cycle in the scalp is reduced to a few weeks or months (inner cycle) at the expense of the anagen phase, resulting in an abundance of miniaturized hair.
Both the normal and pathological hair cycles are subject to multiple environmental factors that have a bearing on their course and homeostasis. In addition to the androgens, the main inducers of hair growth and development and of hair loss in the scalp, hormones, cytokines of the immune system, growth factors, neural activity, nutrition, seasonal changes and aging modulate the action of the androgens and influence the life of the hair.22,23 Certain studies have demonstrated that transforming growth factor type two (TGF-b2,) produced in the dermal papilla by androgenic stimulation, might be one of the factors involved in AGA that mediate the androgenic effect on scalp hair loss via its inhibiting capacity. Insulin-like growth factor 1 (IGF-1) is also expressed in the dermal papilla stimulating hair growth.
CIRCULATING ANDROGENS IN MEN
Testosterone is the most potent androgen in man and its main source of production are the testes. The testes secrete 95% of the 4-9.5 mg of testosterone produced daily, the remaining 5% deriving from the peripheral transformation of the adrenal androgens to testosterone. The testes also secrete approximately 1mg of Δ4-androstendione (Δ4Α) and small quantities of DHEA. The adrenal secretion of androgens consists in the secretion of 10-20mg of dehydro-epi-androsterone (DHEA) and its sulphate DHEA-S, 2-4mg of Δ4-androstendione and minimal amounts of testosterone.
In the blood, normal values of testosterone concentration present a broad range of 3-10 ng/ml, indicating a great inter-individual variation of its production. Testosterone circulates bound to the sex hormone binding globulin (SHBG) and to a lesser degree to albumin and only 1-2% remains free and capable of entering the cells to exert its androgenic action through its transformation into dihydrotestosterone (DHT). Free testosterone (FT) blood concentrations, being influenced by the levels of SHBG, also present a broad range of values. DHEA-S levels in blood are extremely high, 1,000-4,000ng/ml, compared to the other androgenic steroids, because of a slow clearance. DHEA and Δ4A levels are 1,000 times smaller 2.5-7ng/mL and 2-4mg/mL, respectively (Table 1).26-28 The androgenic action of adrenal androgens, which also circulate in both bound and free form, is mediated through successive transformations into the active dihydrotestosterone. DHEA is reversibly transformed into DHEA, 5-7% of which are irreversibly converted to Δ4-androstendione: a percentage of 13-15% of Δ4Α gives rise to testosterone, which is irreversibly transformed mainly intracellularly to dihydrotestosterone at a proportion of 5%.
The skin possesses the enzymatic armament to perform the above transformations of the androgenic steroids that arrive with the circulation as well as those that are synthesized by its own cells. More specifically, dermal papilla cells, a projection of the epidermis into the base of the hair in close connection with the bulb, and the bulb itself express all the above steroids (Figure 4). The necessary enzymes for the expression of the androgenic receptors, the essential factor for conveyance of the androgenic action to the DNA, are also expressed in the dermal papilla and the hair bulb.
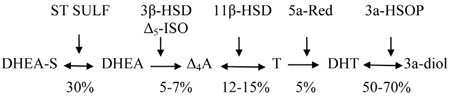
Figure 4. The catalyzing enzymes and the proportion of androgen transformations (ST SULF: Steroid Sulfatase, 3β-HSD: 3β-hydroxysteroid dehydrogenase, Δ5-ISO: Δ5-isomerase, 11β-HSD: 11β-hydroxysteroid dehydrogenase, 5a-Red: 5a-reductase, 3a-HSDR: 3a-hydroxysteroid oxide reductase, 3a-diol: 3a-androstane 17β diol).
THE EFFECT OF ANDROGENS ON THE HAIR FOLLICLE
The evaluation of an androgenic effect on a sensitive tissue requires the comprehensive consideration of all the parameters that are involved in this process, their complex qualitative and quantitative interrelationships and the degree of their participation and contribution to the final effect. This constitutes a difficult task, further confounded by the present scarcity of knowledge and many conflicting results; it is however rewarding because of the amount of vigorous research into the subject.29,30
The chain of events leading to androgenic action are comprised of: the production of testosterone by its principal source, the testes, the peripheral production of testosterone by its precursors, Δ4A, DHEA and DHEA-S, the unknown significance of paracrine and endocrine action of locally produced testosterone by the dermal papilla and the hair bulb, which may be important, the intervention of transcriptional factors, growth factors and cytokines and, finally, a) the local conversion of testosterone to active DHT, b) the normality, functionality and activity of the androgen receptor and c) the modulating action of the coregulators, the three last steps being the most important and studied. Of the former steps, the production of testosterone can be easily measured by its concentration in the blood, whereas their biological activity remains difficult to assess. The transcriptional importance of growth and other factors is discussed in the section on the coregulators.
THE 5A-REDUCTASE
The important role of 5a-reductase in the action of the androgens is demonstrated by the fivefold increase of testosterone androgenic potency through its transformation into dihydrotestosterone and is clinically obvious in subjects with syndromes of 5a-reductase deficiencies who fail to develop hair in a very sensitive target tissue of the androgens, the skin.
5a-reductase reduces the unsaturated bond in the 4-5 position of testosterone’s molecule to form dihydrotestosterone. It can also convert Δ4-androstendione and progesterone to their respective reduced forms. 5a-reductase is expressed in two molecular forms each encoded by a separate gene. The distribution of types 1 and 2 5a-reductase, their ratio and the intensity of their expression varies in the different areas influencing the androgenic effect analogically. Type II 5a-reductase expression is higher in the dermal papilla of scalp hair enhancing the harmful effect of the androgens. Its important role in AGA is demonstrated by the efficacy of finasteride, an inhibitor of type II 5a-reductase, in the treatment of androgenetic alopecia. Also, the few cases of the rare syndrome of 5a-reductase insufficiency that have been reported did not have scalp hair loss.
Biopsies from frontal and occipital sites in 12 men and 12 women with early onset baldness (aged 18-33 years) revealed that both men and women have higher levels of type 1 and 2 5a-reductase in the frontal follicles than in occipital follicles. Women had 3-3.5 times less 5a-reductase in the frontal sites than men.31
THE ANDROGEN RECEPTOR
The androgen receptor (AR) plays a key role in enhancing androgenic activity. The normal expression, structure, function, sensitivity, number and activity of the androgen receptors are essential for the expression and impact of an androgenic effect and any defect in these qualities of the androgen receptor may have significant phenotypic and biological implications, ranging from a simple under- or over-androgenization to complete absence of androgenic action, as is the case in androgen insensitivity syndrome.32
The expression of the androgen receptor is encoded by a gene located in the long arms of chromosome X, at X q11-q12. The AR gene contains 8 coding exons. In the large exon 1, towards the 5΄ end, there is a CAG triplet repeat region that is highly polymorphic and by its extension and variations serves as a polymorphic genetic marker for inheritance patterns of X-chromosome AR defects. The association of the androgen receptor gene with androgen insensitivity and baldness is demonstrated in the case of Kennedy’s disease, which is a neurodegenerative disease provoking spinal bulbar muscular atrophy, testicular atrophy and decreased virilization.33 Kennedy’s disease is caused by an extension of the CAG triplets to 40-70 in number in the gene of the androgen receptor instead of the normal number of 12-35. Australian researchers found that the baldness score was highly significantly smaller in 115 members of the Kennedy’s Diseases Association compared with the score of controls from the Maryborough study.
The androgen receptor is a protein of q10-q19 amino acids belonging to a large family of transcriptional factors that includes the estrogens, the thyroid hormones, glucocorticoid, mineralocorticoid, progesterone receptors and others. The AR protein is comprised of four relatively discrete functional domains, a large amino-terminal domain containing regions involved in transcriptional regulation, a DNA-binding domain in the center of the AR with the characteristic loops of cysteines bound by zinc ions, the zinc fingers, that recognize the binding sequence of DNA and stabilize the DNA-receptor complex, the hinge domain that is assumed to facilitate conformational changes induced by the binding with the androgens and the ligand-binding domain which forms a sort of cavity in which it interacts with the androgen molecule.
The transcriptional action of the androgen receptor involves a complex coordination of molecular interactions. The AR is located in the cellular cytoplasm bound to the heat shock proteins (HSP) which give the AR molecule stability and an optimal conformation for binding to the androgen ligand. The free androgens arriving through the circulation and those produced in situ bind to the binding domain of AR, activating the stabilizing HSPs and the acquirement of a molecular structure that enables the androgen-receptor complex to dimerize and translocate to the nucleus to bind to the hormone response element of DNA. The stimulatory action of the androgen-receptor complex to DNA is modulated by a variety of coregulators that may activate the transcriptional process, the coactivators, or inhibit the corepressors. Several coregulators have been demonstrated in the dermal papilla where they are assumed to play a role in androgenetic alopecia. The affinity of AR is higher for DHT, 5-6 times greater than for testosterone and low for the adrenal androgens DHEA and DEA. The androgen receptor is widely distributed in all skin compartments with varying expression and density in the different anatomic sites. Higher levels of androgen receptors were demonstrated in dermal papilla cells from balding follicles compared with non-balding follicles (p<0.01).34
THE ANDROGEN RECEPTOR COREGULATORS
The last step of androgenic action, the interaction of the androgen-receptor complex with the androgen responsive area of DNA for the realization of its transcriptional activity, is modulated by a variety of proteins that are called coregulators. The coregulators, which are classified as coactivators when they enhance the transcriptional process and corepressors when they inhibit the expression of genes, add another significant factor to the complexity of androgenic action on hair and its paradoxical effect on scalp hair. Moreover, the perplexity increases by the fact that coregulators interact among themselves, enhancing or reducing their potency (see reviews in references35-37).
Coregulators influence the normal transcriptional activity of all the steroid hormone receptors and their mutations or differences of potency may have important implications leading to the development of diseases. Androgen insensitivity syndrome is an example of coregulator contribution to the manifestation of a pathological condition. The mutation in the DNA-binding domain of the androgen receptor prevents an interaction with the coregulators which is essential for the expression of the transcriptional action.
The number of identified coregulators is increasing in animal experiments that are carried out parallel to the vigorous research into their significance in human physiology and the relationship of steroid hormones with cancer. Development of therapeutic strategies targeting the action of coregulators might have an application in scalp hair loss.
GENETIC ASSOCIATIONS OF BALDNESS
Genetic involvement in the pathogenesis of male androgenic alopecia, anticipated because of its well known familial incidence, is indisputable although not to date clearly understood. The high incidence of AGA long pointed to a polygenic mode of inheritance, and this has now been fully established. Research was first aimed at investigation of a potential AGA genetic association with the androgen receptor, as gene mutations of the AR had been described in various diseases, including androgen insensitivity syndrome and prostate cancer. The first demonstration of the link of androgen receptor with AGA was the identification of a single nucleotide polymorphism (SNP) in exon 1 of the AR which is found in almost 100% of men with AGA. However, this SNP is present in a considerable number of non-bald men and, moreover, it is not a coding SNP. In a recent (2011) comprehensive meta-analysis of the association of androgen receptor gene polymorphisms with AGA, Zhuo et al38 evaluated 65 potentially relevant studies and selected 8 that fitted the inclusion criteria, the study comprising 2,074 subjects with AGA and 1,115 controls. The conclusion of this meta-analysis was that the G allele of the AR Stul polymorphism might be a potential risk factor for AGA, though no obvious association was observed between CAG and GGC triplet repeat polymorphisms of the AR gene and the risk for AGA.
Genetic tests for the prediction of baldness have been developed and commercialized based mainly on the genotyping of the androgen receptor variants and, more specifically, on the SNP in exon 1 of the AR, in spite of the lack of specificity of these parameters. In recent years, the pursuit of candidate genes has been continuous both in the X chromosome area and in autosomal chromosomes and in genes expressing DHT, 5a-reductase or other involved enzymes and factors revealing polygenic AGA inheritance. From an epidemiological survey of 9,000 men in a secluded area in Sardinia, a cohort of 200 men was selected with severe AGA of at least Type IV and with onset before the age of 30.39 Case-control association analysis on the X chromosome with the use of the 500K chip array identified a strong association of Xq11-q12 and EDA2R area genes with androgenetic alopecia. A genome-wide study was conducted in 391 individuals of 95 German families with the purpose of identifying new susceptibility genes in AGA.40 The focus with the strongest evidence of linkage was mapped to chromosome 3q26. A larger genome-wide study in 1,125 men from four distinct European populations (Switzerland, United Kingdom, Netherlands, Iceland) demonstrated a newly associated locus at chromosome 20p11.22.41 One man in seven who harbored risk alleles at both 20 p11.22 and the androgen receptor had sevenfold increased odds of androgenetic alopecia. The association of chromosome 20 locus with baldness was replicated in a study of 1,195 men and evidence was found of the association of baldness with two independent loci upstream and downstream of the androgen receptor.42
ANDROGENETIC ALOPECIA COMORBIDITIES
Organs and tissues that are androgen-dependent, like the prostate, the cardiovascular system and others, share common responsive elements in their genes strongly influenced by androgens. The prostate more particularly is in many pathophysiological aspects associated with hair, the common denominator being the androgens. Clinically, prostatic development in embryonic life and at puberty is stimulated by the androgens, while in eunuchs the prostate remains underdeveloped. Similarly, body hair growth at puberty in both sexes is dependent on the action of androgens and, in the case of androgen deficiency, the body is hairless. Scalp hair is not androgen-dependent for its development; however, after puberty its cycle of regeneration is influenced by the androgens. Moreover, at molecular level androgenic action, both in scalp hair and the prostate, is quantitatively more intense through the greater expression of 5a-reductase and of the androgen receptors. Therapeutic trials in AGA, benign prostate hyperplasia and prostate cancer have a common target, minimization of the androgenic effect, and are carried out via the same pharmaceutical agent, finasteride.
The many observed clinical and biological similarities stimulated investigation into the genetic connections between pathological conditions involving scalp hair and the prostate. Identification of a strong association via observation of baldness decades before the clinical expression of prostate cancer was hypothesized to potentially serve as a predictor of its occurrence.
The association between baldness and clinical prostate cancer was studied prospectively in a cohort of 4,421 United States men 25-75 years old who were followed over 17-21 years.43 Incidence of prostate cancer was greater among men with baldness (17.5 versus 12.5 per 10,000 person-years, relative risk 1.50 (1.12-2.00). The risk was independent of race and age. The authors of this paper criticized the five prior studies that had not identified a significant association between AGA and prostate cancer on account of their small sample size and methodological limitations. A population-based case-control study of 1,446 cases of prostate cancer and 1,390 controls in Australia showed no association of frontal baldness with prostatic cancer.44 However, there was a positive association between prostate cancer and vertex baldness.
The association of prostate cancer risk with 5a-reductase type 2 polymorphisms, serum androgens, testosterone, DHT, 3a-androstane-diol glucoronide (3a-diol Gl), DHEA-S, Δ4Α, estradiol, SHBG and androgenetic alopecia was studied in 827 cases of prostate cancer and 736 controls.45 It was observed that carriers of the A49T polymorphism of 5a-reductase present opposite risks for prostate cancer and AGA, the prostate risk being 60% higher and the AGA risk 50% lower. 3a-diol Gl, the main metabolite of DHT considered to be an index of its production, exhibited 35% lower circulating levels. The authors tentatively suggested that if 3a-diol Gl is indeed a marker of DHT production, its low concentration lends weight to the historically less accepted “androgen decline hypothesis” of prostate cancer risk.
A Greek case-control study of the risk factors for prostate cancer in 320 patients with prostate cancer showed no evidence for a positive association between vertex baldness and prostate cancer.46 Evaluating the differences of baldness prevalence, total and free testosterone, FSH, LH, estradiol, prolactin and SHBG in 44 patients with prostate cancer and 108 patients with benign prostate hyperplasia, Feydaci et al found no difference in these parameters between the two patient groups.47
Equally crucial as the potential link between prostate cancer and AGA is the possibility that AGA may be associated with cardiovascular diseases (CVD): such an implication, however, would be one of far greater scope and complexity. The link between AGA and prostate is a case of two parameters, whereas one between AGA and CVD would involve not only myocardial infarction (MI) and death from heart disease but also a wide spectrum of other risk factors including hypertension, hyperinsulinemia, lipid disorders and the Metabolic Syndrome. A strong association of AGA with one of the main risk factors for CVD could well define AGA as a significant prognostic marker of the abovementioned consequences.
Two studies in the 90’s with positive results supported the notion of AGA’s relation to heart disease. The extent and progression of baldness was assessed twice in 2,013 men from Framington, Massachusetts, USA, with a time interval of six years, and the cohort was followed for 30 years for CVD and deaths.48 The extent of baldness was not associated with heart disease; however, the rate of progression (mild, moderate, rapid) was associated with heart disease and mortality. The authors concluded that rapid hair loss might be a marker for coronary heart disease. Lasko et al49 in 1993 published the results of a large case-control study in a sample of 772 men with a first non-fatal myocardial infarction. Vertex baldness (RR 1.4) and severe vertex baldness (RR 3.4) but not frontal baldness alone were associated with myocardial infarction. Ford et al,50 recruiting 2,019 men aged less than 55 years from the 1st National Health and Nutrition Examination study, found that severe baldness was positively associated with ischemic heart disease mortality (RR: 2.51) and somewhat less with ischemic heart disease incidence (RR: 1.72). A retrospective cohort of 22,071 male physicians were asked which of the scale sketches of baldness best approximated their status at 45 years of age. Compared with controls, those with frontal baldness had a relative risk of 1.09, while those with mild, moderate and severe baldness had an RR of 1.23, 1.32 and 1.35, respectively.51
A comprehensive review in 2001 by Rebora52 of the previous literature identified 15 articles dealing with coronary artery disease and baldness: of these, 9 had concluded that there is a relationship between the two conditions, especially in younger subjects with severe early-onset androgenetic alopecia. The controversies among these studies were discussed in another review in 2005 bearing the eloquent title: Is baldness bad for the heart?53
In a more recent (2008) large case-control cross-sectional study, Shahar et al,54 motivated by Lesko’s findings, examined 5,056 men for pattern and degree of baldness and a potential association with prevalence of MI. The estimated odds ratio for MI was 1.28 for frontal baldness, 1.02 for mild vertex baldness, 1.40 for moderate baldness and 1.18 for severe vertex baldness. In addition, the mean differences of carotid internal-medial thickness between men with AGA and controls were close to zero. The authors suggest that male pattern baldness is not a surrogate measure of an important risk factor for myocardial infarction or asymptomatic atherosclerosis. In 2010, a strong relation between cardiovascular risk factors and early onset (<35 years) baldness was identified in 40 men 36-55 years old and 40 controls.55 Metabolic syndrome was diagnosed in 60% of the men with baldness versus 12.5% in controls (OR 10.5), atheromatous plaques were observed in 32% versus 7.5% (OR 5.93) and significantly higher levels of aldosterone and insulin were also found. This publication included 37 women with baldness and 37 controls in whom baldness was strongly associated with metabolic syndrome (48.6% versus 8.1%, OR 10.7) and with carotid atheromatosis (27% versus 8.1%, OR 4.19). Aldosterone, insulin, fibrogen and C-Reactive Protein (CRP) were also significantly higher in women with baldness.
The inconsistent and controversial findings result from the difficulty in assessing and comparing multifactorial relationships between a series of heart risk factors and the types, degree and age of onset of AGA in men and women.
FEMALE BALDNESS, FEMALE ANDROGENETIC ALOPECIA AND FEMALE PATTERN HAIR LOSS
In the early studies of baldness when scalp hair loss was associated with androgenic action it was assumed that this condition etiologically was the same in both sexes and the term androgenetic alopecia was used to characterize female baldness in equivalence to that of men.6 After this title had held sway for many years, it was however realized that female scalp hair loss clinically, etiologically and genetically was in many aspects different from the corresponding features in men, this establishing that baldness in women is a separate entity.56,57 It was thus designated Female Pattern Hair Loss (FPHL), a term that has since been adopted in scientific terminology. Studies on female baldness have been limited in view of the fact that most authors and co-authors of relevant publications have long been women. The fact that female baldness is much less widespread and evident may be another cause of this omission, which has happily been amended over the last few years.
The first significant difference between baldness in women and men is the appearance of head hair in the two sexes. The female pattern of hair loss on the scalp is quite different from that of men. Completely hairless areas on the scalp, which are the hallmark of male baldness, one that is very noticeable and a frequent cause of suffering, are extremely rare in women. In women there is a rarefication and thinning of the hair on the vertex of the scalp, accentuated in the frontal part, and in severe cases bitemporal regression of the frontal line of hair which, if extensive, should arouse suspicion of an androgen-producing tumor. Moreover, estrogens are more involved in the baldness of women, while genetic associations of scalp hair loss are also different in the two sexes. However, the greatest difference between the two sexes is the endocrine milieu, which in woman is characterized by low androgenic v. high estrogenic action, the which latter is abruptly diminished in menopause.
THE INCIDENCE OF BALDNESS IN WOMEN
Limited data exist on the prevalence and types of baldness in Caucasian female populations. In his detailed description and categorization of male baldness, Hamilton had included 214 women.6 The prevalence of Type IV to VIII baldness in these women reached a peak of 20-30% in the 5th decade and did not increase thereafter (data from Figure 2). Norwood,58 whose classification of baldness types is used as a standard, examined 1,006 women and found that the incidence of baldness in women aged 20-29 years was 3%, in those aged 30-49 year it was 16-17%, in the ages 50-69 it was 23-25% and in the ages 70-80 and over 28-32%. The author points out that none of the women had any evidence of excess testosterone. It is presumed that this observation is based on clinical examination. In 327 women aged 18-99 years examined by Birch et al,59 the prevalence of female pattern hair loss was 8% in women aged under 50 years increasing to 38% in subjects 70 years and over. A high incidence of women with FPHL was observed in Maryborough, Australia.12 Sixty-four percent of women aged 80 years and over had bitemporal hair loss and 57% mid-frontal loss.
In contrast, the prevalence and characteristics of baldness in Asiatic women is well documented (Figure 5). In a study of 4,601 Korean women, the prevalence of baldness was low, increasing with age from 0.2 to 3.8% until the 5th decade, to 7.4% in the 6th, 11.7% in the 7th and 24.7% over 70 years of age.12 Ludvig grade I was most common up to the 6th decade and grades I and III over 60 years. Total baldness was not observed. A population-based study of 3,537 Chinese women showed that the prevalence of baldness was even lower than that of Korean women.15 Up to the 7th decade, the prevalence was 0.3 to 3.3%, and 15.4% over 70 years. Similar results were reported in another large study of 8,446 women in 6 Chinese cities.16 The prevalence of baldness was 2.3 to 7.5% until the age of 59 years. 10.3% in women 60-64 years old and 11.8% in those aged 70 years and over. Very low incidence of baldness was also found in 26,226 Taiwanese women aged more than 30 years.60 In women 36-49 years old, the prevalence was 6.3 to 9.9%, in women 50 to 64 years old 11.7% and in those aged 65 to 80 years and over 12.6 to 14.6%.

Figure 5. Incidence of Female Pattern Hair Loss in women at various decades in Caucasian (solid line) and Asiatic (broken line) large population series (1. Hamilton6, 2. Norwood56, 3. Birch57, 4. Gan12, 5. Paik14, 6. Xu15, 7. Wang16, 8. Su60).
GENETIC ASSOCIATION OF FEMALE BALDNESS
Fewer genetic association studies on female baldness have been performed compared with those on male baldness, leaving the problem of baldness inheritance in women virtually unexplored.61 No association between the androgen receptor polymorphisms and Type II pattern of FPHL was determined in scalp biopsies performed in 30 premenopausal Egyptian women aged 39±7 years and in 11 age-matched controls.62 A mild association between the new susceptibility locus at chromosome 20p11 reported by Richards et al41 was found in 397 women from Iceland with types of baldness (OR 1.29, 1.05-1.57) and in 85 British women (OR 1.13, 0,82-1.56). A study of 21 types 1 and 2 of 5a-aromatase isoforms variants, the estrogen receptors 1 and 2 and progesterone receptor was performed in 145 UK and 53 German women with FPHL, without any of these parameters showing a significant association with baldness.63 Investigation of SNPs in and around the CYP19A1 gene encoding 5a-aromatase in 484 Caucasian women with FPHL and 471 controls produced evidence suggesting that the common allele of this gene, which has been associated with higher estrogen levels, might be associated with a predisposition to FPHL.64
THE ENDOCRINE PROFILE OF SEX HORMONES IN WOMEN
The endocrine system in women produces the same steroid hormones as in men, but quantitatively in very different proportions and with very different actions. The mature female organism, while poor in androgenic activity, is flooded by successive waves of estrogens and progesterone during the reproductive years. Estradiol is the dominant estrogen in women that creates the female phenotype at puberty and is essential in the process of ovulation. At around the 51st year of age it vanishes, depriving the female organism of its multiple physiological actions for the rest, that is for 1/3, of the woman’s lifetime.
The secretion of estradiol (E2) during the menstrual cycle is reflected in the measurable levels of E2 in the blood, which are low in the first days of the cycle ranging from 40 to 60 pg/ml and reach a peak of 350-450 pg at the middle of the cycle. The ovary also secrets estrone (E1), a weak estrogen that has a similar secretory pattern to that of E2 but at lower concentrations of 30-200 pg/ml. The cessation of the reproductive and endocrine function of the ovaries at menopause results in a dramatic diminution of estradiol secretion, its serum levels dropping to less than 20pg. Estrone deriving from Δ4Α continues to have measurable blood levels of 30-70 pg/mL after the menopause, but its greatly diminished estrogenic action is demonstrated by the inability of its production to prevent atrophy of the vaginal epithelium, which is a very sensitive tissue requiring minimal estrogenic action. When therefore we speak of estrogens in women, we mean the action of estradiol (Table 2).26,65,66
Two intracellular receptors of estrogens encoded by different genes have been identified, estrogen receptor α(ERα) and β(ERβ), that bind estradiol for triggering of estrogenic action at the nucleus. ERα and ERβ are widely distributed but are differentially expressed in the various tissues. Non-genomic action of the estrogen through membrane receptors is also very probable. Through the abundance of estrogen receptors, estradiol exerts favorable effects on the thickness, collagen content, hydration, elasticity and aging manifestations of the skin.67
Estrogen receptors are expressed in the derma papilla, the hair and the root sheaths of hair. However, it has not as yet been determined whether the action of estrogens on scalp hair is stimulatory or inhibitory. Clinical and experimental observations have provided evidence of both actions.
Progesterone (PG) secretion in the follicular phase of the cycle and in anovulatory cycles is small, the blood levels ranging from 0.5 to 1.5 ng/L. After ovulation, a surge of progesterone occurs during the luteal phase producing levels of 12-15 ng/ml of PG. The impact on the skin and the hair of the 300-350 progesterone secretory waves occurring during the reproductive life of a woman is not known.
Androgenic activity in women derives mainly from Δ-androstendione (Δ4Α) through its transformation into the potent testosterone. The daily production of 2-3mg of Δ4A originates in equal parts from the ovaries and the adrenals, and a smaller proportion from the peripheral transformation of DHEA to Δ4Α. Half of dehydroepiandrosterone is secreted by the adrenals, 25% by the ovaries and the remaining 25%, from peripheral transformation of DHEA-S, is produced exclusively by the adrenals.
Testosterone production is 0.300mg daily, that is 20 times smaller than that of men, and its androgenic activity is further diminished by the fact that the active free testosterone is comparatively low in women because of the increased levels of SHBG that keep bound a great proportion of total testosterone.
This is the androgen profile in women during reproductive life, from puberty until, on average, the 51st year of their life when a dramatic change in estrogenic activity occurs overturning the estrogen/androgen ratio. The distribution in women of androgen receptors, 5a-reductase and 5a-aromatase in the pilosebacious unit and the other androgen-dependent organs is normal and ready to express androgenic activity if it is stimulated by an increase of the endogenous or exogenous androgens. Slight endogenous hyperandrogenemia provokes development of hirsutism and the other skin consequences, while androgen-producing tumors induce male degree mascularization and scalp hair loss much more quickly than naturally occurring baldness in women. These examples of nature are the answer to the query: Are androgens necessary for diffuse hair loss?68
Sex hormone binding globulin (SHBG) is a glycoprotein produced by the liver stimulated by the estrogens. SHBG is a homodimer but contains a single binding site for estrogens and androgens. SHBG production, influenced by the function of the liver, is strongly stimulated by estrogens and inhibited by androgens and insulin. Insulin resistance and obesity are inversely associated with SHBG. Variations of SHBG concentration in the blood are accompanied by inverse changes of the levels of active free estradiol and testosterone.
Women spend the period after the menopause, which may be 1/3 of their lifetime, in a state of multiple endocrine insufficiency. Estrogenic activity that had favorably influenced many functions of the female organism, including the brain, have vanished, adrenal androgens DHEA and DHEA-S are reduced by 50-70%, testosterone is also diminished and the secretion of growth hormone, which in women is the strongest metabolic hormone, is reduced by 50% at 50 years and onward.69 Although it is reasonable to believe that this alteration of the endocrine environment will have an impact on scalp hair, concurrently occurring age-related changes of hair density and other hair features constitute a confounding factor and an obstacle to the correct evaluation of baldness in postmenopausal women.70
TREATMENT OF BALDNESS
While complete restoration of baldness cannot be achieved, a considerable degree of prevention and improvement can be brought about via medical and surgical treatments. Finasterid and minoxidil are today the two available medical treatment options, while hair transplantation is a procedure that yields more permanent and esthetic results.71-73
Finasteride is a selective 5a-reductase isoenzyme 2 inhibitor. It inhibits the conversion of testosterone to dihydrotestosterone, reducing androgenic activity in all androgen-dependent tissues. It has been used in the treatment of benign prostate hypertrophy and prostate cancer. Finasteride, at a dose of 1 mg daily, reduces serum DHT by 60%, reflecting a decrease of androgenic activity in scalp hair. Long-term finasteride treatment can stabilize vertex and frontal hair loss and, to a lesser degree, can stimulate mild to moderate regrowth of scalp hair. Side-effects are rare (0.7 to 1.8%) and include decreased libido, erectile dysfunction and decreased ejaculate volume. Finasteride can decrease PSA in older men.74
Datasteride, an inhibitor of both types of 5a-reductase, is believed to be more efficient than finasteride and certain studies have shown better results. However, it is too early to evaluate correctly the beneficial effects and side-effects of this drug.
Minoxidil was originally used as an oral antihypertensive drug which provoked hypertrichosis as a side-effect. It is used in 2-5% solutions as a local preparation applied to the skull. The efficacy of minoxidil has been demonstrated in 30-35% of men who showed hair growth and moderately increased hair density. After discontinuation of treatment, the hair returns to the basal condition. Side-effects of minoxidil include contact dermatitis (6.5%) and, in women, facial hirsutism (3-5%). Although minimal amounts of minoxidil are absorbed, patients with hypotension or tachycardia should consult the cardiologist before using the drug. Certain patients prefer combined therapy with both finasteride and minoxidil.
Surgical hair restoration techniques have made considerable advances. Hair follicles from the scalp occipital area that are relatively androgen-resistant are transplanted to balding areas providing natural and permanent hair. Transplantation is an invasive and costly procedure but very effective in the hands of a dexterous and expert operator.75
Laser or light preventive and therapeutic treatments for baldness have become very popular in recent years, with several laser machines and light sources being available for use in clinics or at home.76 However, while there is much interest in this kind of treatment, specification of the impact of wavelengths and other details have not yet been defined by large-scale investigations.
REFERENCES
1. Hamilton JB, 1960 Effect of castration in adolescent and young adult males upon further changes in the proportions of bare and hairy scalp. J Clin Endocrinol Metab 20: 1309-1318.
2. Cash TF, 1992 The psychological effects of androgenetic alopecia in men. J Am Acad Dermatol 26: 926-931.
3. Wells PA, Willmoth T, Russell RJ, 1995 Does fortune favour the bald? Psychological correlates of hair loss in males. Br J Psychol 86: 337-344.
4. Lee HJ, Ha SJ, Kim D, Kim HO, Kim JW, 2002 Perception of men with androegenetic alopecia by women and nonbalding men in Korea: How the nonbald regard the bald. Int J Dermatol 41: 867-869.
5. Budd D, Himmelberger D, Rhodes T, Cash TE, Girman CJ, 2000 The effects of hair loss in European men: a survey in four countries. Eur J Dermatology 10: 122-127.
6. Hamilton JB, 1951 Patterned loss of hair in man: Types and incidence. Ann NY Acad Sci 53: 708-728.
7. Norwood OT, 1975 Male pattern baldness: Classification and incidence. South Med J 68: 1359-1365.
8. Severi G, Sinclair R, Hopper JL, et al GG, 2003 Androgenetic alopecia in men aged 40-69 years: Prevalence and risk factors. Brit J Dermatology 149: 1207-1213.
9. Rhodes T, Girman CJ, Savin RC, 1998 Prevalence of male pattern hair loss in 18-49 year old men. Dermatol Surg 24: 1330-1332.
10. DeMuro-Mercon C, Rhodes T, Girman CJ, Vatten L, 2000 Male-pattern hair loss in Norwegian men: a community-based study. Dermatology 200: 291-222.
11. Birch MP, Messenger AG, 2001 Genetic factors predispose to balding and non-balding in men. Eur J Dermatology 11: 309-314.
12. Gan DC, Sinclair RD, 2005 Prevalence of male and female hair loss in Maryborough. J Investig Dermatol Symp Proc 10: 184-189.
13. Tang PH, Chia HP, Cheong LL, Koh D, 2000 A community study of male androgenetic alopecia in Bishan, Singapore. Singapore Med J 41: 202-205.
14. Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI, 2001 The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol 145: 95-99.
15. Xu F, Sheng YY, Mu ZL, et al, 2009 Prevalence and types of androgenetic alopecia in Shanghai, China: a community-based study. Br J Dermatol 160: 629-631.
16. Wang TL, Zhou C, Shen YW, 2010 Prevalence of androgenetic alopecia in China: A community-based study in six cities. Br J Dermatol 162: 843-847.
17. Pathomvanich D, Pongratananukul S, Thienthaworn P, Manoshai S, 2002 A random study of Asian male androegenetic alopecia in Bangkok, Thailand. Dermatol Surg 28: 804-807.
18. Lee WS, Lee HJ, 2012 Characteristics of androgenetic alopecia in Asian. Ann Dermatol 23: 243-252.
19. Courtois M, Loussouarn G, Hourseau C, Grollier JF, 1999 Hair cycle and alopecia. Skin Pharmacol 7: 84-89.
20. Messenger AG, 2011 Hair through the female life cycle. Br J Dermatol 165: Suppl 3: 1-6.
21. Randall VA, 2007 Hormonal regulation of hair follicles exhibits a biological paradox. Semin Cell Dev Biol 18: 274-289.
22. Chen CC, Chuong CM, 2012 Multi-layered environmental regulation on the homeostasis of stem cells: the saga of hair growth and alopecia. J Dermatol Sci 66: 3-11.
23. Randall VA, Ebling FJ, 1991 Seasonal changes in human hair growth. Br J Dermatol 124: 146-151.
24. Hibino T, Nishiyama T, 2004 Rolf of TGF-β2 in the human hair cycle. J Dermatol 35: 9-18.
25. Inui S, Itami S, 2011 Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla. J Dermatol Science 61: 1-6.
26. Nafziger AN, Bowlin SJ, Jenkins PL, et al, 1998 Longitudinal changes in dehydroepiandrosterone concentrations in men and women. J Lab Clin Med 131: 316-323.
27. Kaufman JM, Vermeulen A, 1998 Androgens in male senescence. In: Nieschlag E, Behre HM (eds), Springer Verlag Berlin; pp,437-471.
28. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, 2001 Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 86: 724-731.
29. Zouboulis CC, Degitz K, 2004 Androgen action on human skin - from basic research to clinical significance. Exp Dermatol 13: Suppl 4: 5-10.
30. Alsantali A, Shapiro J, 2009 Androgens and hair loss. Curr Opin Endocrinol Diabetes Obes 16: 246-253.
31. Sawaya ME, Price VH, 1997 Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol 109: 296-300.
32. Quigley CA, 1998 The androgen receptor: Physiology and pathophysiology. In: Nieschlag E, Behre HM (eds), Testosterone Action Deficiency Substitution, Springer Verlag Berlin; pp, 83-106.
33. Sinclair R, Greenland KJ, van Egmond V, 2007 Men with Kennedy disease have a reduced risk of androgenetic alopecia. Br J Dermatol 157: 290-294.
34. Hibberts NA, Howell AE, Randall VA, 1998 Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol 156: 59-65.
35. McKenna NJ, Lanz RB, O’ Malley BW, 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20: 321-344.
36. Heinlein CA, Chang C, 2002 Androgen receptor (AR) coregulators: An overview. Endocrine Reviews 23: 175-200.
37. Thakur MK, Paramanik V, 2004 Role of steroid hormone coregulators in health and disease. Horm Res 71: 194-200.
38. Zhuo FL, Xu W, Wang L, 2011 Androgen receptor gene polymorphisms and risk for androgenetic alopecia: a meta-analysis. Clin Exper Dermatol 37: 104-111.
39. Prodi DA, Pirastu N, Maninchedda G, 2008 EDA2R is associated with androgenetic alopecia. J Invest Dermatology 128: 2268-2270.
40. Hillmer AM, Flaquer A, Hanneken S, 2008 Genome-wide scan and fine-mapping linkage study of androgenetic alopecia reveals a locus on chromosome 3q26. Amer J Human Genetics 82: 737-743.
41. Richards JB, Yuan X, Geller F, 2008 Male-pattern baldness susceptibility locus at 20p11. Nat Genet 40: 1282-1284.
42. Cobb JE, Zaloumis SG, Scurrah KJ, Harrap SB, Ellis JA, 2010 Evidence for two independent functional variants for androgenetic alopecia around the androgen receptor gene. Exp Dermatol 19:1026-1028.
43. Hawk E, Breslow RA, Graubard B, 2000 Male pattern baldness and clinical prostate cancer in the epidemiologic follow-up of the first National Health and Nutrition Examination Survey. Cancer Epidemiol Biom 9: 523-527.
44. Giles GC, Severi G, Sinclair R, 2002 Androgenetic alopecia and prostate cancer: Findings from an Australian case-control study. Cancer Epidemiol Biomarkers Prev 11: 549-556.
45. Hayes VM, Severi G, Padilla EJD, 2006 5a-reductase type 2 variant associations with prostate cancer risk, circulating hormone levels and androgenetic alopecia. Int J Cancer 120: 776-780.
46. Hsieh CC, Thanos A, Mitropoulos D, et al, 1999 Risk factors for prostate cancer: a case-control study in Greece. Int J Cancer 80: 699-703.
47. Faydaci G, Bilal E, Necmettin P, 2008 Baldness, benign prostate hyperplasia, prostate cancer and androgen levels. The Aging Male 11: 189-192.
48. Herrera CR, D’ Agostino RB, 1995 Baldness and coronary heart disease rates in men from the Framingham study. Amer J Epidemiol 142: 828-833.
49. Lesko SM, Rosenberg L, Shapiro S, 1993 A case-control study of baldness in relation to myocardial infarction in men. JAMA 269: 998-1003.
50. Ford ES, Freedman DS, Byers T, 1996 Baldness and ischemic heart disease in a national sample of men. Am J Epidemiol 143: 651-657.
51. Lotufo PA, Chae CU, Ajani UA 2000 Male pattern baldness and coronary heart disease: The physicians’ Health Study. Arch Intern Med 160: 165-171.
52. Rebora A, 2001 Baldness and coronary artery disease. Arch Dermatol 137: 943-947.
53. Cutersohn T, Scheidegger EP, 2005 Is baldness bad for the heart? Dermatology 74: 1399-1406.
54. Shahar E, Heiss G, Rosamond WD, Szklo M, 2008 Baldness and myocardial infarction in men. The atherosclerosis risk in communities study. Am J Epidemiol 167: 676-683.
55. Arias-Santiago S, Gutierrez-Salmeron MT, Castellote-Caballero L, 2010 androgenetic alopecia and cardiovascular risk factors in men and women: A comparative study. J Am Acad Dermatol 63: 420-429.
56. Birch MP, Messenger JF, Messenger AG, 2001 Hair density, hair diameter and the prevalence of female pattern hair loss. Br J Dermatol 144: 297-304.
57. Ludwig E, 1977 Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol 97: 247-254.
58. Norwood OT, 2001 Incidence of female androgenetic alopecia (female pattern alopecia). Dermatol Surg 27: 53054.
59. Birch MP, Messenger JF, Messenger AG, 2001 Hair density, hair diameter and the prevalence of female pattern hair loss. Br J Dermatol 144: 297-304.
60. Su LH, Li-Sheng C, Hsiu-His C, 2012 Factors associated with female pattern hair loss and its prevalence in Taiwanese women: A community-based survey. J Am Acad Dermatol 69: e69-77.
61. Yip L, Rufaut R, Sinclair R, 2011 Role of genetics and sex steroid hormones in male androgenetic alopecia and female pattern hair loss: An update of what we now know. Austr Dermatol 52: 81-88.
62. El-Samahy MH, Shaheen MA, Saddik DE, 2009 Evaluation of androgen receptor gene as a candidate gene in female androgenetic alopecia. Int J Dermatol 48: 584-587.
63. Redler S, Tazi-Ahnini R, Drichel D, 2012 Selected variants of the steroid-5-alpha-reductase isoforms SRD5A1 and SRD5A2 and the sex steroid hormone receptors ESR1, ESR2 and PGR: No association with female pattern hair loss identified. Exp Dermatol 21: 390-393.
64. Yip L, Zaloumis S, Irwin D, 2009 Gene-wide association study between the aromatase gene (CYP19A1) and female pattern hair loss. Brit J Dermatol 161: 289-294.
65. Longcope C, 1986 Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab 15: 213-228.
66. Burger HG, Dudley EC, Cui J, 2006 A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab 85: 2832-2838.
67. Zouboulis CC, Chen WC, Thornton MJ, Rosenfield R, 2007 Sexual hormones in human skin. Horm Metab Res 39: 85-95.
68. Orme S, Cullen DE, Messenger AG, 1999 Diffuse hair loss: are androgens necessary? Br J Dermatol 141: 521-523.
69. Batrinos ML, 2012 The aging of the endocrine hypothalamus and its dependent endocrine glands. Hormones (Athens) 11: 241-253.
70. Mirmirani P, 2011 Hormonal changes in menopause: do they contribute to a “midlife hair crisis” in women? Br J Dermatol 165: Suppl 3: 7-11.
71. Blumeyer A, Tosti A, Messenger A, Reygagne P, 2011 Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges 9: Suppl 6: 1-57.
72. Miteva M, Tosti A, 2012 Treatment options for alopecia: An update, looking to the future. Expert Opin Pharmacother 13: 1271-1281.
73. Blume-Peytavi U, Atkins S, Gieler U, Grimalt R, 2012 Skin academy: hair, skin, hormones and menopause – current status / knowledge on the management of hair disorders in menopausal women. Eur J Dermatol 22: 310-318.
74. Otberg N, Finner AM, Shapiro J, 2007 Androgenetic alopecia. Endrocrinol Metab Clin N Am 36: 379-398.
75. McElwee KJ, Shapiro J, 2012 Promising therapies for treating and/or preventing androgenic alopecia. Skin Therapy Lett 17: 1-4.
76. Rangwala S, Rashid RM, 2012 Alopecia: a review of laser and light therapies. Dermatol Online J 18: 3.
Address for correspondence:
Menelaos L. Batrinos, 8 Evripidou Str., Politeia-Kifissia, 14563, Greece, Tel.: +30 210 6204041, E-mail: mbatrinos@gmail.com
Received: 28-11-2013, Accepted: 06-02-2014