1Clinical Endocrinology, Charit Campus Mitte, Charitί University Medicine Berlin; 2Department of Endocrinology and Metabolism, Medizinische Klinik-Innenstadt, University Hospital Munich; 3Endocrinology & Diabetes Unit, Department of Internal Medicine I, University Hospital of Wrzburg, Werzburg; 4Endokrinologikum, Berlin; Germany
OBJECTIVE: In subjects at high risk for sleep apnea (SA), aldosterone concentrations correlate with severity of SA and primary aldosteronism (PA) is very often diagnosed. Patients with PA show a high prevalence of SA. Treatment of PA either by adrenalectomy (ADX) or mineralocorticoid receptor (MR) blockade is thought to abolish the increased comorbidities. However, no data are available regarding effectiveness of different PA treatments on quality of sleep. DESIGN: This prospective multi-center study included 15 patients with newly diagnosed PA evaluated before and 0.7±0.2 years after treatment initiation, and a second cohort including 81 patients who were evaluated 5.3 and 6.8 years after treatment initiation. Biochemical parameters, 24h blood pressure and three validated self-assessment questionnaires (Giessen Complaint List (GBB-24), Epworth Sleepiness Scale (ESS) and Pittsburgh Sleep Quality-Index (PSQI)) were analyzed. RESULTS: Z-scores of exhaustion tendency of GBB significantly improved in newly diagnosed PA patients after treatment initiation (1.8±1.4 vs. 1.0±1.2, p=0.034). In the second cohort no differences were found in GBB-24, ESS and PSQI. No differences were found in all three questionnaires independently of type of PA therapy. However, female patients scored significantly higher than males in the PSQI (8.7±3.6 vs 5.7±4.2, p<0.005), indicating lower sleep quality, independently of the type of therapy. CONCLUSIONS: For the first time, we analyzed quality of sleep in patients with PA, demonstrating that therapy initiation improves exhaustion tendency. Surprisingly, female PA patients showed significantly more sleep disturbances than male PA patients several years after treatment initiation.
Adrenalectomy, Aldosterone, Eplerenone, Hyperaldosteronism, Sleep quality, Spironolactone
INTRODUCTION
Many studies have shown a connection between obstructive sleep apnea (OSA) and hypertension, especially resistant hypertension, and have demonstrated that it affects more men than women, and particularly obese subjects.1-5 Untreated sleep apnea leads to excessive daytime sleepiness (EDS) and can also result in insulin resistance, obesity and stroke.6,7 Interestingly, high aldosterone levels are a common finding among patients with sleep disorders.1,8,9 Several studies 3,10 point to OSA as a trigger of the sympathetic activity via hypoxia-induced chemoreceptor stimulation and aldosterone/sodium retention followed by an increased edema of nasopharyngeal tissue and oxidative stress as well as endothelial dysfunction. Mineralocorticoid receptor (MR) blockade seems to be effective for the treatment of OSA,5,11 this supporting the hypothesis of MR involvement and endothelial dysfunction. Sleepiness cannot be measured directly as it is a subjective complaint;12 however, a common symptom of bad sleep is EDS. Patients with EDS are at high risk for OSA because of sleep fragmentation and nocturnal hypoxemia.13,14
Primary aldosteronism (PA) is the most common form of secondary hypertension and affects up to 10% of all hypertensive patients,15-19 and even up to 28% in patients with resistant hypertension.1,3,10,20 Patients with PA have an increased risk of developing relevant comortalities and comorbidities, e.g. vascular, cardiac or cerebrovascular morbidities and renal insufficiency.16,21-24 Data from the German Conn’s registry showed an elevated prevalence (6.7%) of OSA in PA patients.16 Up to now, no data is available regarding sleep quality and EDS in patients with aldosterone excess such as PA. It is also unknown if abolishment of aldosterone excess by blockade of MR or by adrenalectomy (ADX) improves sleep quality and EDS.
Therefore, the aim of this study was a) to investigate sleep quality in PA patients by using self-reporting established questionnaires, b) to investigate possible changes in sleep quality due to therapy initiation in PA patients and c) to detect differences in sleep quality regarding the type of PA therapy and gender.
SUBJECTS AND METHODS
Design and patients
The German Conn’s Registry (www.conn-register.de) is a multi-center database analyzing comorbidities and long-term outcome of patients with PA.16,17,25 Since the initiation of the prospective phase in October 2008, all patients actively treated within the centers were entered into a common database after pseudonymization.26 The Ethics Committees of the University of Munich and of the participating centers approved the protocol. Data protection laws were strictly adhered to.
Clinical data at time of diagnosis were extracted from patients’ charts, including laboratory test results, initiation of mineralocorticoid antagonist treatment, surgical treatment, cardiovascular comorbidities, body mass index (BMI) and metabolic conditions. In the case of multiple determinations, the measurements of potassium, plasma renin concentration and aldosterone at first presentation were used for statistical calculations. Blood was generally drawn in the fasting state, although this was not standardized among centers. Every six and twelve months, the patients were seen for follow-up visits, including a clinical examination, complete laboratory investigation, cardiovascular examinations and self-reporting established questionnaires.
The diagnostic criteria for PA in this study were chosen according to the Endocrine Society Practice guidelines.27 All patients included had an elevated aldosterone to renin ratio (ARR) and an abnormal confirmatory test (saline infusion test, fludrocortisone suppression test, captopril test or oral salt loading test with demonstration of elevated excretion of aldosterone and its metabolites in urine).16,17 Adjustment of medication prior to screening and confirmation was performed whenever possible with beta-blockers, central alpha-2 agonists, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, as well as diuretics withdrawn for at least one week and mineralocorticoid antagonists for at least four weeks. The diagnosis of PA was centrally verified by review of all available data.
Whole cohort:
Three hundred and thirteen (313) patients with PA were registered since 2008 in the prospective phase of the German Conn’s Registry in the three largest centers. Only patients with a complete data set were included. Sufficient data coverage was available for 109 prospectively treated patients (34.8%), between 2008 and August 2011, who were included in the final analysis (Munich, n=63; Berlin, n=38; Würzburg, n=10). Subtype differentiation between aldosterone producing adenoma (APA) and bilateral adrenal hyperplasia was based on adrenal imaging (computed tomography or magnetic resonance imaging). In addition, adrenal vein sampling was performed in 54% to 87% of the patients in the participating centers.28 For further analysis, we distinguished between two patients cohorts in our prospective cohort:
Prospective cohort of newly diagnosed PA patients (cohort 1):
Newly diagnosed PA patients (since October 2008; cohort 1) were included and evaluated prior to start of therapy (pretreatment) and followed up thereafter (follow-up). Of the 109 newly diagnosed PA patients, complete data of 15 patients were available including pretreatment and 12-month follow-up visits. Unilateral adrenalectomy (ADX) was performed in 11 of 15 patients (73.3%) for suspected unilateral aldosterone excess, mainly APA. The remaining non-operated four patients were treated with different medical regimens: spironolactone (n= 3; 20%) with a mean dose of 41.6±14.4mg/d (mean±SD), (range 25-50mg/d), or other antihypertensives (n=1; 6.7%).
Prospective cohort of diagnosed PA patients on therapy (cohort 2):
Two hundred and four (204) PA patients (diagnosis of PA before October 2008; cohort 2) were included in the study after initiation of therapy had already started and were evaluated during long-term follow-up at two outpatient visits (called visits V1 and V2). Of these 204 patients, 96 patients had a complete clinical data set of follow-up visits V1 and V2, which were approx. 1.4±0.6 years (mean±SD) apart. Questionnaires were completely answered by 81 patients (85.4%). Unilateral adrenalectomy (ADX) was performed in 39 patients (40.6%) for suspected unilateral aldosterone excess, mainly APA. The remaining non-operated 57 patients were treated with different medical regimens: spironolactone (n= 39; 40.6%), eplerenone (n=13; 13.5%), or other antihypertensives (n=5; 5.3%).
Self-reporting questionnaires:
a) Epworth Sleepiness Scale (ESS)
The ESS measures the subject’s general level of daytime sleepiness,29 more specifically, the sleep propensity which is the ratio of total sleep drive to total wake drive.30 The questionnaire describes eight daily life situations, which are rated regarding their probability of dozing off on a scale from 0 to 3 (highest chance).31 The total score is the sum of the eight questions and can range from 0 to 24, with a higher score indicating higher sleep propensity. The cut-off for increased sleep propensity is ten.
b) Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a retrospective questionnaire for the last four weeks.32 It consists of seven components like sleep quality, sleep latency and sleep-inducing drug consumption. Patients rate 18 questions on a 4-point scale from 0 to 3. Items are related to one of the seven components named above. A score ≥5 has a high sensitivity and specificity for indicating sleep disturbances and those patients are regarded as poor sleepers.33,34
c) Giessen Complaint Questionnaire (GBB-24, Giessener Beschwerdebogen)
The short form of GBB-24 evaluates such physical complaints as exhaustion tendency, stomach trouble, rheumatic pains and heart trouble.35 For our analysis we used only the six items related to exhaustion tendency. Each item is answered on a 5-point scale ranging from never to always: the higher the scores, the higher the exhaustion tendency. Adjustment for age and sex was performed by transformation of score values into age (decade) and sex-adjusted z-scores. Calculation of z-scores was based on the complete data set from the respective normative group for the GBB-24 (n= 2076).36
Statistics
Variables were assessed for normality by the Kolmogorov-Smirnov test. Results are expressed as mean ± standard deviation (SD) if not stated otherwise. Differences between the two groups were assessed using Student’s t-test for normally distributed variables and the Mann-Whitney test for non-normally distributed variables. For paired data (cohort 1) we used the paired Wilcoxon test. We used the Kruskal-Wallis test when the examined groups were of unequal size. A p-value<0.05 was considered as significant. Statistical analysis was carried out using IBM SPSS Statistics 20.
RESULTS
Cohort 1
In this prospective cohort, 15 patients completed the GBB-24 (7 women, 8 men; age: 48.6 ± 10.4 years, range: 30 - 66 years), 13 subjects completed the ESS (6 women, 7 men; age: 48.9 ± 10.9 years, range: 30 - 66 years) and only two patients completely filled in the PSQI at diagnosis of PA (before treatment initiation) and 0.74 ± 0.22 years afterwards under therapy. Due to insufficient patient numbers we excluded the PSQI in this cohort.
At diagnosis of PA the patients had had arterial hypertension for 10.1±9.8 years. After treatment initiation, the systolic and diastolic blood pressures decreased significantly, also night time blood pressure values were lowered considerably (Table 1). Furthermore, potassium levels increased and aldosterone levels and the aldosterone-renin ratio (ARR) dropped (Table 1). Between pretreatment and follow-up visit we could not see any significant changes in the total ESS score but z-scores of exhaustion tendency in the GBB-24 improved significantly (1.8±1.4 vs. 1.0±1.2, p=0.034, Figure 1).

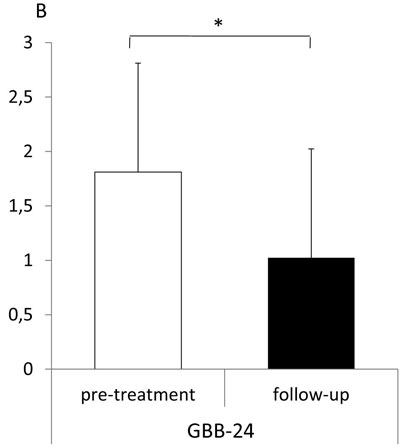
Figure 1. Epworth Sleepiness Scale (ESS, A) and exhaustion tendency of the Giessen Complaint Questionnaire (GBB-24, B) in patients with PA before (pretreatment, white bars) and 1.0 ± 0.1 years after initiation of treatment (follow-up, black bars) (cohort 1). Higher scores mean worse outcome. ESS cutoff >10 indicates increased sleep propensity. Means ± SD., *= p<0.05. GBB-24 z-score adjusted values (± SD).
Cohort 2
Eighty-two (82) patients (31 women, 51 men) with a mean age of 60.8 ± 10.5 years (range: 22-80 years), who were already on therapy for PA diagnosed 5.3 ± 3.6 years before study inclusion, were evaluated in a prospective fashion during long-term follow-up. At visit 1 the patients had a BMI of 29.2 ± 5.1 kg/m2 and had had arterial hypertension for 18.5 ± 11.2 years, which was well controlled (24h systolic/diastolic BP during day 132/81 ± 11/9 mmHg; 24h systolic/diastolic BP during night 119/71 ± 15/10 mmHg) with 2.3 ± 1.9 antihypertensive drugs. Serum potassium levels were normal (4.2 ± 0.4 mmol/l). Analysis of cohort 2, as a representative “follow-up cohort” of cohort 1, showed that patients of cohort 2 were significantly older and had had hypertension for a longer period of time. However, BMI and the male:female ratio among patients was not significantly different between the two cohorts.
During long-term follow-up of cohort 2, patients’ blood pressure and potassium levels remained unchanged within the normal range; only the number of antihypertensive drugs increased significantly to 2.6 ± 2.3 (p<0.05). No significant differences in all three questionnaires (ESS, PSQI, and GBB-24) occurred during follow-up of cohort 2 (data not shown); however, PSQI scores were high, indicating poor sleep quality (data not shown). In addition, no differences in all three questionnaire scores were seen between patients on MR-antagonist therapy and patients who received ADX (data not shown), or between MR-antagonists spironolactone (72.2 ± 58.9 mg/day (range 50-240)) and eplerenone (87.5 ± 48.3 mg/day (range 25-200)) (data not shown). Patients receiving MR-antagonist treatment had the same blood pressure, BMI and potassium levels, but had higher aldosterone levels (361.9 ± 260.4 vs 98.1 ± 112.7 ng/l; p<0.001) and a higher aldosterone to renin ratio (ARR) (34.3 ± 48.4 vs 9.7 ± 13.5; p<0.01) than patients who received ADX.
Analysis of differences between the sexes in cohort 2 showed that the women were significantly younger (56.7 ± 12.3 vs 63.5 ± 8.2 years; p<0.05) and leaner (26.9 ± 5.4 vs 30.5 ± 4.5 kg/m2; p<0.01) than the men. They also showed significantly lower systolic night time blood pressure levels (114 ± 13 vs 123 ± 73 mmHg; p<0.01) and less antihypertensive medications (1.5 ± 1.9 vs 2.8 ± 1.8; p<0.001) than men. However, women showed significantly higher scores in the PSQI, indicating a significantly poorer sleep quality than men (Figure 2). This effect was also seen in women who underwent adrenalectomy and who were on MR-antagonist treatment (data not shown).
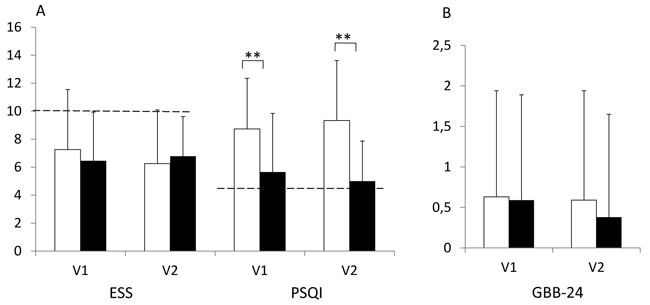
Figure 2. Epworth Sleepiness Scale (ESS, A), Pittsburgh Sleep Quality-Index (PSQI, A) and Giessen Complaint Questionnaire (GBB-24, B) in female (white bars) and male (black bars) patients with PA after initiation of treatment during long-term follow-up (study visits V1 and V2) (cohort 2). Higher scores mean worse outcome. ESS cut-off >10 indicate increased sleep propensity; PSQI cut-off ≥5 indicate sleep disturbances. Means ± SD. ** = p<0.005. GBB-24 z-score adjusted values (±SD).
DISCUSSION
Recently Calhoun et al. showed that subjects at high risk for sleep apnea were almost two times more likely to have PA diagnosed and had a higher 24h-urinary aldosterone excretion.9 In a further study they observed that a significant correlation existed between plasma aldosterone concentration and the severity of obstructive sleep apnea (OSA), this suggesting that aldosterone excess may contribute to obstructive sleep apnea severity.37 Interestingly, we found a high prevalence (6.7%) of sleep apnea in a large retrospective cohort of patients with PA in Germany.16 This led to the question of sleep quality in PA patients.
In this prospective study with patients with PA, we showed that initiation of therapy resulted in a significant improvement of exhaustion tendency. Sleep quality assessed by three questionnaires remained stable over several years after treatment initiation of PA. For the first time we demonstrated that there is no difference between adrenalectomy and MR antagonist therapy regarding sleep quality in PA patients.
A previous study showed that treatment with MR antagonists results in an improvement of symptoms of sleep apnea, indicating a possible correlation between OSA and fluid retention which leads to oro-pharyngeal edema and hence to sleep apnea.1 This might be also a pathophysiological process in patients with PA. In addition, poor sleep quality, expressed as higher PSQI scores, was observed in non-dippers with newly diagnosed stage 1 hypertension compared to dippers,38 suggesting that loss of blood pressure decline during the night might be involved. Typically, PA patients have a non-dipping blood pressure profile.24
Poor sleep quality is also associated with greater psychosocial distress.39 Therefore, some diseases such as diabetes mellitus are associated with excessive daytime sleepiness.40 Interestingly, it is reported that diabetes mellitus occurs more often in PA patients than in control persons.25
In our study we detected a sex-specific difference with regard to PSQI scores, indicating a worse sleep quality in women with treated PA. Several studies have addressed gender differences in answering quality of life and sleep quality questionnaires and showed controversial results.33,41 Backhaus et al33 described a possible shift in PSQI scores that may be attributed to memory distortion and focus on bad nights. In the Sleep Heart study, women and men reported their feeling to the same extent, but fewer women had a total ESS score >10.41 Baldwin et al. suggested that male gender reporting and the severity of the ESS score correlated more strongly with unrest and sleepy feelings. Another known gender difference is upper airway resistance which is greater in men than women.42 One might hypothesize that this would result in worse sleep quality in men. However, we detected worse PSQI scores for women compared to men. In addition, men were older than women in our cohort. These differences need to be addressed in further studies.
Limitations of our prospective study include possible differences in patients and data handling between participating centers, although they should be minor because of prior standardization and agreement on diagnostic protocols. In addition, irregular sleep-wake rhythms (shift work, jet lag) were not directly asked for. Furthermore, sleep was assessed only by self-reported questionnaires and no objective measures were performed.
In conclusion, we analyzed quality of sleep in patients with PA, demonstrating that therapy initiation improves exhaustion tendency. The type of PA therapy seems not to be relevant. We suggest that there are sex-specific differences regarding sleep quality in PA patients which need to be further investigated.
ACKNOWLEDGEMENTS
We are indebted to Kathrin Zopf, Christiane Friedrich, Gregor Hanslik and Verena Fourkiotis, all of Clinical Endocrinology, Charité Campus Mitte, University Medicine Berlin, for their help with patients’ recruitments. We also express our gratitude to the other centres of the study for their collaboration.
REFERENCES
1. Gonzaga CC, Gaddam KK, Ahmed MI, et al, 2010 Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med 6: 363-368.
2. Gus M, Goncalves SC, Martinez D, et al, 2008 Risk for Obstructive Sleep Apnea by Berlin Questionnaire, but not daytime sleepiness, is associated with resistant hypertension: a case-control study. Am J Hypertens 21: 832-835.
3. Pedrosa RP, Drager LF, Gonzaga CC, et al, 2011 Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension 58: 811-817.
4. Lloberes P, Lozano L, Sampol G, et al, 2010 Obstructive sleep apnoea and 24-h blood pressure in patients with resistant hypertension. J Sleep Res 19: 597-602.
5. Dudenbostel T, Calhoun DA, 2012 Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens 26: 281-287.
6. Stevenson JE, 2003 Diagnosis of sleep apnea. WMJ 102: 25-7, 46.
7. Drager LF, Genta PR, Pedrosa RP, et al, 2010 Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol 105: 1135-1139.
8. Di MA, Petramala L, Cotesta D, et al, 2010 Renin-angiotensin-aldosterone system in patients with sleep apnoea: prevalence of primary aldosteronism. J Renin Angiotensin Aldosterone Syst 11: 165-172.
9. Calhoun DA, Nishizaka MK, Zaman MA, Harding SM, 2004 Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest 125: 112-117.
10. Pimenta E, Calhoun DA, Oparil S, 2009 Sleep apnea, aldosterone, and resistant hypertension. Prog Cardiovasc Dis 51: 371-380.
11. Gaddam K, Pimenta E, Thomas SJ, et al, 2010 Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens 24: 532-537.
12. Mathis J, Hess CW, 2009 Sleepiness and vigilance tests. Swiss Med Wkly 139: 214-219.
13. Araujo SM, Bruin VM, Daher EF, Medeiros CA, Almeida GH, Bruin PF, 2011 Quality of sleep and day-time sleepiness in chronic hemodialysis: a study of 400 patients. Scand J Urol Nephrol 45: 359-364.
14. Chen R, Xiong KP, Lian YX, et al, 2011 Daytime sleepiness and its determining factors in Chinese obstructive sleep apnea patients. Sleep Breath 15: 129-135.
15. Quinkler M, Stewart PM, 2010 Treatment of primary aldosteronism. Best Pract Res Clin Endocrinol Metab 24: 923-932.
16. Born-Frontsberg E, Reincke M, Rump LC, et al, 2009 Cardio- and cerebrovascular comorbidites of hypo- and normokalemic primary aldosteronism: results of the German Conn’s Registry. J Clin Endocrinol Metab 94: 1125-1130.
17. Reincke M, Rump LC, Quinkler M, et al, 2009 Risk Factors Associated with a Low Glomerular Filtration Rate in Primary Aldosteronism. J Clin Endocrinol Metab 94: 869-875.
18. Rossi GP, 2004 Primary aldosteronism: a needle in a haystack or a yellow cab on Fifth Avenue? Curr Hypertens Rep 6: 1-4.
19. Rossi GP, Bernini G, Desideri G, et al, 2006 Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension 48: 232-238.
20. Acelajado MC, Calhoun DA, 2011 Aldosteronism and resistant hypertension. Int J Hypertens 20: 837817. doi: 10.4061/2011/837817.
21. Born-Frontsberg E, Reincke M, Beuschlein F, Quinkler M, 2009 Tumor size of Conn’s adenoma and comorbidities. Horm Metab Res 41: 785-788.
22. Quinkler M, Born-Frontsberg E, Fourkiotis VG, 2010 Comorbidities in Primary Aldosteronism. Horm Metab Res 42: 429-434.
23. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ, 2005 Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 45: 1243-1248.
24. Fourkiotis V, Vonend O, Diederich S, et al, 2012 Effectiveness of eplerenone or spironolactone treatment in preserving renal function in primary aldosteronism. Eur J Endocrinol 168: 75-81.
25. Reincke M, Meisinger C, Holle R, et al, 2010 Is Primary Aldosteronism Associated with Diabetes Mellitus? Results of the German Conn’s Registry. Horm Metab Res 42: 435-439.
26. Fischer E, Beuschlein F, Bidlingmaier M, Reincke M, 2011 Commentary on the Endocrine Society Practice Guidelines: Consequences of adjustment of antihypertensive medication in screening of primary aldosteronism. Rev Endocr Metab Disord 12: 43-48.
27. Funder J, Carey R, Fardella C, et al, 2009 Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. Eur J Endocrinol [Epub ahead of print].
28. Vonend O, Ockenfels N, Gao X, et al, 2011 Adrenal venous sampling: evaluation of the German Conn’s registry. Hypertension 57: 990-995.
29. Johns MW, 1991 A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14: 540-545.
30. Johns MW, 1993 Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest 103: 30-36.
31. Johns MW, 1994 Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep 17: 703-710.
32. Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ, 1989 The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28: 193-213.
33. Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F, 2002 Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 53: 737-740.
34. Fiorentini A, Valente R, Perciaccante A, Tubani L, 2007 Sleep’s quality disorders in patients with hypertension and type 2 diabetes mellitus. Int J Cardiol 114: E50-E52.
35. Brahler E, Scheer JW, 1979 [Scaling of psychosomatic by means of the Giessen inventory (GBB) (author’s transl)]. Psychother Med Psychol (Stuttg) 29: 14-27.
36. Brahler E, Schumacher J, Brahler C, 2000 [First all-Germany standardization of the brief form of the Gissen Complaints Questionnaire GBB-24]. Psychother Psychosom Med Psychol 50: 14-21.
37. Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA, 2007 Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest 131: 453-459.
38. Erden I, Erden EC, Ozhan H, et al, 2010 Poor-quality sleep score is an independent predictor of nondipping hypertension. Blood Press Monit 15: 184-187.
39. Suarez EC 2008 Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain Behav Immun 22: 960-968.
40. Vgontzas AN, Bixler EO, Chrousos GP, 2003 Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med 254: 32-44.
41. Baldwin CM, Kapur VK, Holberg CJ, Rosen C, Nieto FJ, 2004 Associations between gender and measures of daytime somnolence in the Sleep Heart Health Study. Sleep 27: 305-311.
42. Schmidt-Nowara WW, Coultas DB, Wiggins C, Skipper BE, Samet JM, 1990 Snoring in a Hispanic-American population. Risk factors and association with hypertension and other morbidity. Arch Intern Med 150: 597-601.
Address for correspondence:
Marcus Quinkler, MD, Clinical Endocrinology, Charité Campus Mitte, Charité University Medicine Berlin Charitéplatz 1, D 10117 Berlin, Germany Tel.: ++49-30-450514152; Fax: ++49-30-450514958 e-mail: marcus.quinkler@charite.de
Received 28-04-2013, Accepted 16-09-2013