1Division of Endocrinology and Metabolism, Department of Internal Medicine, 2Department of Vascular Surgery, 3Department of Orthopedics, 4Department of Infectious Diseases, 5Department of Plastic and Reconstructive Surgery, 6Department of General Internal Medicine, Dokuz Eylul University School, Inciralti, Izmir, Turkey
OBJECTIVE: Prediction of diabetic foot ulcer outcome may be helpful for clinicians in optimizing and individualizing management strategy. The aim of the present study was to examine the possibility of predicting the outcome of patients with diabetic foot ulcers by using easily assessed clinical and laboratory parameters at baseline. DESIGN: In this observational study, data were collected prospectively in 670 consecutive diabetic foot ulcer episodes in 510 patients examined between January 1999 and June 2008 and were used to evaluate potential predictors of amputation retrospectively. After exclusion of patients who did not come to the hospital for follow-up for a minimum of six months, data of 574 foot ulcer episodes were evaluated. RESULTS: Limb ischemia, osteomyelitis and presence of gangrene and ulcer depth, determined by the Wagner classification system, were the major independent predictors of overall and major amputations. Older age, presence of coronary artery disease, smoking and ulcer size were found to be associated with either overall or major amputations. Baseline levels of acute phase reactants (white blood cell count, polymorphonuclear leukocyte count, platelet count, erythrocyte sedimentation rate (ESR), serum C-reactive protein (CRP) and albumin) and decreased hemoglobin levels were associated with amputation risk. Multivariate analysis showed that one standard deviation increase in baseline CRP and ESR levels were independent predictors of overall and major amputations, respectively. CONCLUSIONS: The presence of limb ischemia, osteomyelitis, local and diffuse gangrene and ulcer depth were independent predictors of amputation. Baseline levels of ESR and CRP appeared to be helpful for clinicians in predicting amputation.
Acute phase reactants, Amputation, Diabetes, Foot ulcer, Ischemia, Osteomyelitis
INTRODUCTION
Diabetic foot ulcers are a major reason for lower extremity amputations.1 Fifteen percent of diabetic patients develop foot ulcers during their lifetime and a significant number of individuals with this diabetic complication require a lower extremity amputation.2 Diabetic ulcers precede non-traumatic lower-extremity amputations in 85% of diabetic patients. Furthermore, the risk of lower extremity amputation is 15 to 46 times higher in diabetics than in individuals who do not have diabetes mellitus.1,3,4
Diabetic patients with long-term, inadequately controlled blood glucose levels are at significant risk for serious complications affecting the lower limbs.1 The most common risk factors for ulcer formation are altered foot sensation, foot deformities, trauma, peripheral arterial occlusive disease and previous foot ulcer or amputation.5,6 Despite well defined risk factors in the development of diabetic foot ulcer, there are limited data on factors that predict amputation. Ulcer depth, severity of infection, ischemia, osteomyelitis and gangrene are considered as predictors of amputation in a diabetic foot ulcer. Additional factors that have been linked to amputation include older age as well as macrovascular and microvascular co-morbidities.1,5,7-9
The management of diabetic foot ulcers includes evaluation of vascular status, identification of infection including osteomyelitis, antibiotic therapy, surgical debridement, metabolic control of diabetes and minimal amputation when necessary.2,10 It is very important for clinicians to know which clinical and laboratory findings at admission are associated with poor outcome, namely amputation, in patients with diabetic foot ulcers. Prediction of the outcome in patients with diabetic foot ulcers might be helpful for clinicians in optimizing and individualizing management strategy. The aim of the present study was to determine potential baseline clinical and laboratory factors in predicting the outcome in patients with diabetic foot ulcers.
PATIENTS AND METHODS
Dokuz Eylul University Hospital is a referral centre for diabetic foot problems in the western part of Turkey. In this observational study, data were collected prospectively in 670 consecutive diabetic foot ulcer episodes in 510 patients examined between January 1999 and June 2008 and were used retrospectively to evaluate potential predictors of amputation. To determine ulcer outcome, patients were followed for a minimum period of six months. Ulcers which developed in patients in whom a previous ulcer had healed and another event during the period of follow-up had arisen were classified as new ulcer episodes. Because of the variable capacity of the patients for follow-up visits, 96 ulcer episodes in 88 patients were followed for a period of less than six months and these data were excluded from the analysis. Finally, records of 574 foot ulcer episodes were reviewed. Before the data collection, all patients gave their consent to having their data used for scientific purposes, including a study protocol. Institutional Review Board approval was obtained.
Data regarding patients’ characteristics, medical history including smoking habits and physical examination findings were evaluated. At presentation, a photograph of the ulcer was taken. The site and the largest diameter of the ulcer were noted. Depth of the ulcer was determined by inspection, with additional use of a sterile probe if indicated. Foot lesions were classified according to the Wagner classification. Wagner classification grades were as follows. 1: ulcerated skin and subcutaneous tissue, 2: deeper lesions that could penetrate to tendon, bone or joint capsule (there is as yet no abscess or osteomyelitis), 3: deep tissues are involved, abscess, osteitis or osteomyelitis are present, 4: local gangrene, 5: diffuse gangrene.
Plain X-ray films of affected bones were taken. Magnetic resonance imaging (MRI) of the extremity was performed in selected ulcers (n=117). Baseline glycosylated hemoglobin (A1c), kidney and liver function tests and hemoglobin levels were recorded. Arterial circulation was evaluated by palpation of the peripheral pulses and ankle brachial pressure index (ABI) using a handheld Doppler. Patients with absent or reduced pedal pulses or ABI <0.9 underwent conventional Doppler examination. Patients with vascular insufficiency were evaluated by the vascular surgeon and revascularization procedure was examined if indicated. Conventional or MRI angiography (n=127) was performed in selected patients, especially in patients undergoing vascular surgery or angioplasty. Symptoms of neuropathy were recorded. All of the study subjects were tested for neuropathy using the 10g Monofilament test. Loss of vibration perception was evaluated by a biothesiometer on the pulp of the hallux. Further neurological assessments were carried out when required.
Treatment consisted of daily wound care, bed rest, special materials used to avoid putting pressure on the affected area when ambulating, parenteral antibiotics and debridement or amputation (minor or major) when indicated. Major amputation was defined as an amputation done above the ankle joint. Wound debridement was performed routinely to remove extensive callus and necrotic tissue. Skin grafting was performed if necessary. No growth factor was used in any patient. Infected diabetic foot ulcer was defined according to the Infectious Diseases Society of America guidelines as the presence of purulent wound drainage or ≥3 designated systemic or local inflammatory findings. Samples were obtained for culture by deep-needle aspiration, bone biopsy or curettage of the ulcer. In patients with infected diabetic foot ulcers, antibiotics were given according to the decision of the specialist in infectious diseases. After obtaining culture specimens, empirical parenteral treatment was started; change in antimicrobial regimen was guided by culture results and clinical follow-up. Parenteral antibiotic treatment was followed by prolonged oral therapy.
Electrocardiogram and careful cardiac examinations were performed in all patients and their eyes were examined by an ophthalmologist for diabetic retinopathy. The diagnosis of coronary artery disease was established if the patient had angiographically proven coronary ischemia, history of myocardial in¬farct, coronary angioplasty or coronary artery by-pass grafting. The presence of hypertension was recorded. Subjects having a blood pressure level higher than 140 mmHg for systolic and 90 mmHg for diastolic at examination or patients using antihypertensive drugs were defined as hypertensive. Diabetic nephropathy was investigated via 24 hours urinary albumin excretion and serum creatinine levels.
Levels of acute phase reactants were available at presentation in 386 diabetic foot ulcer episodes. Baseline white blood cell count (WBC), polymorphonuclear leukocyte count (PNL), platelet count (PLT), erythrocyte sedimentation rate (ESR) and serum albumin and C-reactive protein (CRP) levels were evaluated.
STATISTICAL ANALYSIS
Logistic regression was used to estimate the independent effect of each selected variable on the outcome. The association between prognostic variables and major amputation rate was evaluated by calculating the odds ratios and their corresponding 95% confidence intervals. One-way ANOVA was used to compare continuous variables. Differences in proportions were compared using the chi-squared test. Receiver operating characteristic (ROC) curves were generated to determine predictability of levels of acute phase reactants for overall and major amputations. Sensitivity, specificity and positive and negative predictive values for different cut-off levels of ESR and CRP were calculated. Analyses were conducted on SPSS 11.0 software. Mean values are given with ± SD. All tests of significance were 2-tailed. P values less than 0.05 were considered statistically significant.
RESULTS
The average duration of time from presentation with foot ulcer to final categorization as healing, unhealing and amputation was 9.55±2.75 months (9.64±2.51 months in the healed ulcer group, 9.67±2.21 in the unhealed ulcer group and 9.39±3.21 months in the amputation group, p >0.05). Patients who underwent amputation were older and had longer duration of diabetes when compared to patients whose ulcers healed during follow-up (p=0.001, p=0.001, respectively). Patients who underwent amputation more frequently had hypertension and coronary artery disease (p=0.018, p=0.02, respectively). There was a significantly higher number of smokers in the amputation group (p=0.041). BMI was significantly lower in the amputation group (p=0.002). There was a higher incidence of ischemia and osteomyelitis in amputation and unhealed ulcer groups (p <0.05). Among patients with limb ischemia, 27 (26.7%, n=101) in the healed ulcer group, 11 (22.7%, n=48) in the unhealed ulcer group and 53 (30.8%, n=172) in the amputation group underwent revascularization treatment (p >0.05). Neuropathy was less frequent in the amputation group (p <0.001). Patients who underwent amputation had slightly greater ulcer size (p=0.023). The site of ulcers and the Wagner scores are shown in Table 1 . There was higher acute phase response in patients with amputation and unhealed ulcers (p <0.05), while baseline A1c levels were comparable (p >0.05, Table 1 ).
Baseline clinical and laboratory predictors of overall and major amputation were evaluated by univariate logistic regression analysis, the results of which are shown in Table 2 . Older age, presence of coronary artery disease, smoking, limb ischemia, osteomyelitis, increased levels of WBC, PNL, PLT, ESR, CRP and decreased levels of hemoglobin and albumin were found to be associated with greater risk for overall amputations. Neuropathy was associated with decreased odds ratio, possibly due to the fact that some patients with ischemic ulcers, who underwent amputation, had no neuropathy. Predictors of major amputation were smoking, limb ischemia, osteomyelitis, ulcer size, increased values of WBC, ESR, CRP and decreased levels of hemoglobin and albumin. Multivariate logistic regression analysis showed that presence of limb ischemia (OR: 4.021, p=0.001, 95% CI: 1.77 – 9.137), osteomyelitis (OR: 3.821, p <0.001, 95% CI: 1.932 – 7.559) and one standard deviation increase in CRP level (OR: 3.718, p=0.005, 95% CI: 1.489 – 9.284) were associated with increased risk for overall amputations when the data were controlled for age, gender, BMI, duration of diabetes, smoking and presence of coronary artery disease (model r2: 0.285). As for major amputations, presence of limb ischemia (OR: 15.136, p=0.001, 95% CI: 2.852 – 80.321), osteomyelitis (OR: 4.546, p=0.004, 95% CI: 1.605 – 12.871) and one standard deviation increase in ESR level (OR: 3.513, p=0.013, 95% CI: 1.289 – 9.608) were found to be associated with increased risk when the data were controlled for age, gender, BMI, duration of diabetes, smoking and presence of coronary artery disease (model r2: 0.22). Presence of local or diffuse gangrene and increased ulcer depth, defined according to the Wagner grades, were significant predictors of both overall and major amputations (Wagner grade ≥4 vs. Wagner grade ≤3, overall amputations: Model r2: 0.318, OR: 24.975, p <0.001, 95% CI: 11.466 – 54.4; Wagner grade ≥4 vs. Wagner grade ≤3, major amputations: Model r2: 0.166, OR: 11.299, p <0.001, 95% CI: 5.216 – 24.016; Wagner grade 3 vs. Wagner grade ≤2, overall amputations: Model r2: 0.133, OR: 5.036, p <0.001, 95% CI: 2.609 – 9.722; Wagner grade 3 vs. Wagner grade ≤2, major amputations: Model r2: 0.047, OR: 6.797, p <0.001, 95% CI: 1.765 – 26.169; data were controlled for age, gender, BMI, duration of diabetes, smoking and presence of coronary artery disease).
ROC curves were generated to evaluate the relationship between levels of acute phase reactants and overall and major amputations. ESR and CRP levels were more likely to be associated with overall and major amputations according to AUC values which were obtained from ROC curves (Table 3 ). Potential cut-off values of sedimentation rate and CRP were determined as predictors of overall and major amputations (Figure 1). Sensitivity, specificity and positive and negative predictive values for different cut-off levels of ESR and CRP are shown in Table 4 . The cut-off level of 100 mg/dl for CRP seemed to be a better predictor of overall amputation with reasonable PPV and NPV. On the other hand, the cut-off level of 90 mm/h for ESR had fair specificity and reasonable PPV and NPV for the prediction of major amputation. As osteomyelitis was one of the major predictors of amputation in our study, we also evaluated baseline levels of acute phase reactants in diabetic ulcer episodes with and without osteomyelitis. Patients with osteomyelitis had significantly prominent acute phase response when compared to those without osteomyelitis (Table 5 ).


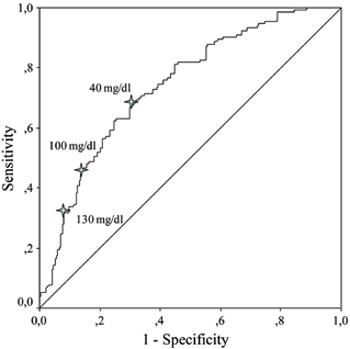
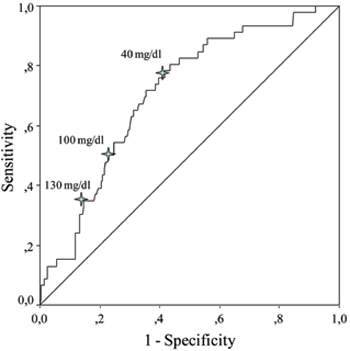
Figure 1. Receiver operating characteristic curves (ROC) showing sedimentation rate and serum C-reactive protein in predicting overall and major amputations.
DISCUSSION
The present study examined whether or not certain baseline clinical and laboratory features can predict the risk for overall and major amputations in a diabetic foot ulcer episode. In particular, the potential relationship between baseline levels of acute phase reactants and amputation risk was systematically evaluated. The studied subjects constitute the largest cohort in the literature with pertinent data. It was shown that limb ischemia, osteomyelitis, presence of gangrene and ulcer depth were major independent predictors of overall and major amputations. The relationship between major amputation rate and the presence of limb ischemia was very strong. A high Wagner grade was another strong predictor of the ulcer outcome. Several other baseline clinical characteristics such as older age, presence of coronary artery disease, smo¬king and ulcer size were found to be associated with either overall or major amputations. Baseline levels of acute phase reactants and decreased hemoglobin levels were associated with increased amputation risk. Multivariate analysis showed that baseline CRP and ESR levels were independent predictors of overall and major amputations, respectively.
Despite well defined risk factors for diabetic foot ulcer development, limited data are available as to which factors predict amputation in a diabetic foot ulcer episode. In concordance with our results, previous studies also showed that limb ischemia and osteomyelitis are associated with an increased risk for amputation.11-13 Eneroth et al14 found that limb ischemia was an independent risk factor for amputation. Diamantopoulos et al15 showed that limb ischemia was the major factor associated with worse outcome in diabetic foot infections. Winkley et al8 reported that mortality was threefold greater in diabetic foot ulcers with ischemia. Depth of the ulcer has been found to be an important predictor of the ulcer outcome.16 Deep ulcerations were reported to be associated with threefold increased risk for amputation.8 Similarly, Eneroth et al14 reported that a diabetic foot wound exposing the bone was more likely to be associated with amputation. A significant association between osteomyelitis and overall and major amputations has been reported.7,17 Armstrong et al17 demonstrated that major amputation risk was 11-fold higher when the wound penetrated to bone. No association between infection and ulcer outcome was reported in a study from Nottingham. Nevertheless, the prevalence of osteomyelitis was very low in this cohort.16 Oyibo et al7 reported that the Wagner grade significantly correlated with the risk of amputation. Calhoun et al18 reported that increased Wagner grade was associated with a higher treatment failure. Ulcers of Wagner grades 4 and 5 denote the presence of local or diffuse gangrene, which are usually due to a combination of ischemia and infection. It is thus not surprising that grade 4 and 5 ulcers were very strongly associated with amputation in our study. To evaluate the effect of ulcer depth on outcome, we calculated odds ratios regarding Wagner grade 3 vs. grade 1 and 2 ulcers. Because of the small number of ulcers with Wagner grade 1, we combined Wagner grade 1 and grade 2 ulcers. We found that increased ulcer depth, defined by the Wagner classification, was an independent predictor of amputation.
We also found that baseline levels of acute phase reactants, especially CRP and ESR, were related to outcome. Similarly, Volaco et al19 found that elevated CRP levels strongly predicted major amputation in longstanding diabetic patients with ischemic foot lesions. In a prospective study, Lipsky et al20 showed that elevated baseline levels of acute phase reactants (WBC, CRP, ESR) were associated with clinical treatment failure in diabetic foot infections treated with broad spectrum antibiotics. Low serum albumin was reported to be associated with increased amputation risk.13 Leukocytosis was related to worse clinical outcomes in diabetic foot ulcer.20,21 A WBC count >12.0 cells/μL was associated with increased risk for amputation.14 On the other hand, Armstrong et al22 found that leukocytosis was a poor indicator of acute osteomyelitis, whereas most patients with osteomyelitis had elevated ESR. In another study conducted by Pittet et al,23 PNL count was not found to be an independent predictor of treatment failure. Although patients who underwent amputation in our study had increased WBC and PNL counts at baseline, neither one of these parameters was an independent predictor according to multivariate analysis.
Acute phase response in diabetic foot ulcer mostly depends on limb ischemia, severity of infection and the presence of osteomyelitis. Elevated ESR has been proposed as a useful diagnostic marker of osteomyelitis when combined with clinical data. In a pilot study, Kaleta et al24 suggested that an ESR level of 70 mm/h might be the optimal cut-off point to predict osteomyelitis with sensitivity of 89.5% and specificity of 100%. In a recent meta-analysis, an ESR level of more than 70 mm/h has been reported to increase the probability of osteomyelitis 11 times.25 Our data suggested that the cut-off level of 90 mm/h for ESR and the cut-off level of 100 mg/dl for CRP had fair specificity and reasonable PPV and NPV for the prediction of major amputations. It has been reported that CRP was increased in hematogenous osteomyelitis in children, and decreased faster than ESR after appropriate treatment, reflecting the effectiveness of therapy with a higher sensitivity than ESR.26 It is also known that patients with peripheral artery disease have increased levels of inflammation markers.27 Elevated levels of CRP have been associated with poor long-term prognosis in patients with peripheral artery disease.28
Our study had some limitations. First, the clinical and laboratory data were collected only at presentation and thus a number of patients may have had various local complications during the course of the ulcer episode which might have affected wound healing. Second, there was no single standard antibiotic therapy for all ulcers. However, antibiotics were given according to the suggestion of an infectious disease specialist. According to our hospital antibiotic therapy guideline, antibiotic treatment was initiated empirically and was revised according to culture results and clinical outcome. Third, some patients underwent revascularization procedures according to the decision of the vascular surgeon, which might have enhanced wound healing. As severe limb ischemia is associated with worse outcome in diabetic foot ulcers,14,15 all patients were evaluated for possible revascularization procedure and revascularization was utilized when considered appropriate. Fourth, the diagnosis of osteomyelitis was made only in a limited number patients using bone histopathology. Another point was that we used only the Wagner classification system. Wagner classification reflects the dominance of gangrene and it is also informative for ulcer depth. However, little information could be obtained as to ulcer size, severity of infection and denervation by Wagner grading. Other classification systems such as University of Texas (UT), Perfusion, Extent/size, Depth/tissue loss, Infection and Sensation (PEDIS), and Size (Area and Depth), Sepsis, Arteriopathy, and Denervation (S(AD)SAD) may provide more detailed information about the ulcer but unfortunately these classifications were not applied in our patients. In conclusion, the presence of limb ischemia, osteomyelitis and increased Wagner grade were major predictors of amputation in our cohort. Our results also suggest that baseline levels of acute phase reactants, especially CRP and ESR, might be helpful in predicting the outcome in patients with diabetic foot ulcers.
DECLARATION OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
1. Jeffcoate WJ, Harding KG, 2003 Diabetic foot ulcers. Lancet 361: 1545-1551.
2. 1999 Consensus Development Conference on Diabetic Foot Wound Care: 7-8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes Care 22: 1354-1360.
3. Armstrong DG, Lavery LA, Quebedeaux TL, Walker SC, 1997 Surgical morbidity and the risk of amputation due to infected puncture wounds in diabetic versus nondiabetic adults. South Med J 90: 384-389.
4. Lavery LA, Ashry HR, van Houtum W, et al, 1996 Variation in the incidence and proportion of diabetes-related amputations in minorities. Diabetes Care 19: 48-52.
5. Most RS, Sinnock P, 1983 The epidemiology of lower extremity amputations in diabetic individuals. Diabetes Care 6: 87-91.
6. Pecoraro RE, Reiber GE, Burgess EM, 1990 Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 13: 513-521.
7. Oyibo SO, Jude EB, Tarawneh I, et al, 2001 A comparison of two diabetic foot ulcer classification systems: the Wagner and the University of Texas wound classification systems. Diabetes Care 24: 84-88.
8. Winkley K, Stahl D, Chalder T, Edmonds ME, Ismail K, 2007 Risk factors associated with adverse outcomes in a population-based prospective cohort study of people with their first diabetic foot ulcer. J Diabetes Complications 21: 341-349.
9. Faglia E, Favales F, Morabito A, 2001 New ulceration, new major amputation, and survival rates in diabetic subjects hospitalized for foot ulceration from 1990 to 1993: a 6.5-year follow-up. Diabetes Care 24: 78-83.
10. Lipsky BA, Berendt AR, Deery HG, et al, 2004 Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 39: 885-910.
11. Reiber GE, Pecoraro RE, Koepsell TD, 1992 Risk factors for amputation in patients with diabetes mellitus. A case-control study. Ann Intern Med 117: 97-105.
12. Mayfield JA, Reiber GE, Nelson RG, Greene T, 1996 A foot risk classification system to predict diabetic amputation in Pima Indians. Diabetes Care 19: 704-709.
13. Flores Rivera AR, 1998 Risk factors for amputation in diabetic patients: a case-control study. Arch Med Res 29: 179-184.
14. Eneroth M, Apelqvist J, Stenstrom A, 1997 Clinical characteristics and outcome in 223 diabetic patients with deep foot infections. Foot Ankle Int 18: 716-722.
15. Diamantopoulos EJ, Haritos D, Yfandi G, et al, 1998 Management and outcome of severe diabetic foot infections. Exp Clin Endocrinol Diabetes 106: 346-352.
16. Treece KA, Macfarlane RM, Pound N, Game FL, Jeffcoate WJ, 2004 Validation of a system of foot ulcer classification in diabetes mellitus. Diabet Med 21: 987-991.
17. Armstrong DG, Lavery LA, Harkless LB, 1998 Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 21: 855-859.
18. Calhoun JH, Cantrell J, Cobos J, et al, 1988 Treatment of diabetic foot infections: Wagner classification, therapy, and outcome. Foot Ankle 9: 101-106.
19. Volaco A, Chantelau E, Richter B, Luther B, 2004 Outcome of critical foot ischaemia in longstanding diabetic patients: a retrospective cohort study in a specialised tertiary care centre. Vasa 33: 36-41.
20. Lipsky BA, Sheehan P, Armstrong DG, et al, 2007 Clinical predictors of treatment failure for diabetic foot infections: data from a prospective trial. Int Wound J 4: 30-38.
21. Akanji AO, Famuyiwa OO, Adetuyibi A, 1989 Factors influencing the outcome of treatment of foot lesions in Nigerian patients with diabetes mellitus. Q J Med 73: 1005-1014.
22. Armstrong DG, Lavery LA, Sariaya M, Ashry H, 1996 Leukocytosis is a poor indicator of acute osteomyelitis of the foot in diabetes mellitus. J Foot Ankle Surg 35: 280-283.
23. Pittet D, Wyssa B, Herter-Clavel C, et al, 1999 Outcome of diabetic foot infections treated conservatively: a retrospective cohort study with long-term follow-up. Arch Intern Med 159: 851-856.
24. Kaleta JL, Fleischli JW, Reilly CH, 2001 The diagnosis of osteomyelitis in diabetes using erythrocyte sedimentation rate: a pilot study. J Am Podiatr Med Assoc 91: 445-450.
25. Butalia S, Palda VA, Sargeant RJ, Detsky AS, Mourad O, 2008 Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA 299: 806-813.
26. Roine I, Faingezicht I, Arguedas A, Herrera JF, Rodriguez F, 1995 Serial serum C-reactive protein to monitor recovery from acute hematogenous osteomyelitis in children. Pediatr Infect Dis J 14: 40-44.
27. Cassar K, Bachoo P, Ford I, Greaves M, Brittenden J, 2005 Markers of coagulation activation, endothelial stimulation and inflammation in patients with peripheral arterial disease. Eur J Vasc Endovasc Surg 29: 171-176.
28. Violi F, Criqui M, Longoni A, Castiglioni C, 1996 Relation between risk factors and cardiovascular complications in patients with peripheral vascular disease. Results from the A.D.E.P. study. Atherosclerosis 120: 25-35.
Address for correspondence:
Baris Akinci, MD, Division of Endocrinology of Metabolism, Department of Internal Medicine, Dokuz Eylul University Medical School, Inciralti, Izmir, Turkey 35340, Tel.: +90-232-4123744; Fax: +90-232-2792267, e-mail: baris.akinci@deu.edu.tr
Received 11-06-09, Revised 10-08-09, Accepted 10-09-09