1Institute of Endocrinology, Diabetes and Diseases of Metabolism, Clinical Center of Serbia, Belgrade, Serbia, 2Endocrine Unit, Evgenidion Hospital, University of Athens, Greece, 3Department of Internal Medicine I, University of Ulm, Germany, 4Department of Medicine, University of Santiago de Compostela, Santiago de Compostela, Spain
*Current Address: Hospital of the Paul-Gerhardt-Foundation, Martin-Luther University, Halle-Wittenberg, Germany
OBJECTIVE: Ghrelin, a potent stimulator of GH secretion, also acts as an orexigenic hormone. Plasma ghrelin levels rise before meals with postprandial reduction, suggesting that circulating levels of enteroinsular hormones might influence ghrelin secretion. AIM: The aim of this study was to evaluate the effects of ghrelin on enteroinsular hormones in healthy men. DESIGN: Three tests were performed on 3 different days in 6 healthy men: On the first day, saline was infused from 0-120 minutes followed by a ghrelin bolus (1 μg/kg) administration (Test 1); on the second experimental day, GHRH was administered at 0 min, and a ghrelin bolus was given at 120 min (Test 2); on the third experimental day, GHRP-6 was administered at 0 min, followed by a ghrelin bolus at 120 min (Test 3). Plasma glucose, insulin, proinsulin, C-Peptide Glucagone Like Peptide one (GLP-1) determined at 0, 15, 30, 60, 90, and 120 min of the test period. RESULTS: There was a significant increase in AUC glucose (526.41±22.91 mmol · ml-1 · min vs. 566.37±15.64 mmol · ml-1 · min; p<0.05) and AUC insulin (756.25±107.56 mU · ml-1 · min vs. 981.62±180.32 mU · ml-1 · min; p<0.05) and a significant decrease in AUC GLP-1 (2346.87±874.28 pmol · ml-1 · min vs. 1769.5±784 pmol · ml-1 · min; p<0.05) after ghrelin administration in Test 1 compared to Test 3. There was a mild but non-significant increase in AUC for insulin, proinsulin, and C-Peptide and a mild reduction in AUC GLP-1 after every ghrelin administration. CONCLUSION: There was no evidence of a direct effect of ghrelin administration on enteroinsular hormone levels in this study. However, ghrelin may potentiate the glucose-insulin stimulatory effects of GHRP-6. More studies should be carried out for further evaluation of ghrelin-enteroinsular hormones interplay.
Enteroinsular hormones, Ghrelin, GLP-1, Insulin
INTRODUCTION
Ghrelin, a 28-amino acid peptide, is secreted primarily by the stomach but also in small amounts by the bowel, kidney, placenta, pituitary, and hypothalamus.1,2 It is an endogenous ligand of the growth hormone (GH) secretagogue receptor type 1a (GHS-R) and one of the most potent stimulators of GH secretion.1,3
The highest concentrations of ghrelin are found in the oxynthic glands of the gastric fundus, while gastrectomy has been reported to reduce ghrelin plasma levels by 65-80%.4,5 Ghrelin also acts as an orexigenic hormone stimulating food intake. It decreases energy expenditure and fat catabolism and modulates, in an age and gender independent manner, the secretory patterns of somatotroph, lactotroph, and corticotroph cells.6-8 Therefore, these findings may indicate a substantial role of the peptide in the control of food intake and energy metabolism. Plasma ghrelin levels rise shortly before meals, followed by a postprandial reduction at 60-120 min, possibly suggesting that circulating enteroinsular hormones, such as insulin, glucagon, Glucagon Like Peptide 1 (GLP-1), or local factors such as stomach distension, might influence ghrelin secretion.10 Clamp studies suggested that hypo- and hyperglycemia do not influence ghrelin secretion, while hyperinsulinemia suppresses plasma ghrelin levels.11 In contrast, other findings demonstrated that ghrelin is suppressed by OGTT-induced hyperinsulinemia and hyperglycemia, thus questioning the direct regulation of its metabolism by insulin and glucose.12 It is noteworthy that only acylated ghrelin is endocrine active, whereas unacylated ghrelin, although 2.5-fold higher in serum, exerts some actions on the cardiovascular system and on adipogenesis.13
It was shown that GLP-1 exerts a small but non-significant inhibition of ghrelin secretion from isolated rat stomach, most probably through an enhancement of somatostatin activity.14 GLP-1, in contrast to ghrelin, induces satiety via a central mechanism in addition to its effects on insulin and glucagon secretion and on gastric emptying.15 During the oral glucose tolerance test, GLP-1 and ghrelin are interrelated in an inverse manner.16
The aim of this study was to further evaluate the complex interrelationship between ghrelin and hormones of the enteroinsular axis. To this end, we measured the levels of glucose, GLP-1, Insulin (INS), Proinsulin (PI), and C-Peptide (CP) during ghrelin, GHRH, and GHRP-6 (a hexapeptide belonging to the family of the synthetic GHRPs which possess a strong stimulatory effect on GH secretion) infusions in healthy men.
SUBJECTS AND METHODS
In six healthy men (age: 32.17±8.42 years, BMI: 22.64±2.01 kg/m2), three double tests were performed on three different days in random order, at least one week apart. After overnight fasting, an intravenous canilla was placed in a forearm vein and tests were started at 08.00h. On the first experimental day, saline was infused from 0-120 min. At 120 min a bolus of ghrelin (Europeptides, Argenteuil, France) was given at the dose of 1 μg/kg (saline-ghrelin test, Test 1). On the second experimental day the subjects were given Growth Hormone Releasing Hormone (GHRH 1-29 NH2, Geref, Serono, Madrid, Spain) at the dose of 1 μg/kg at 0 min. At 120 min a bolus (1 μg/kg) of ghrelin was given (GHRH-Ghrelin test, Test 2). On the third experimental day the subjects were given GHRP-6 (Clinalfa, Laufelfinger, Switzerland) at the dose of 1 μg/kg iv at 0 min followed by ghrelin bolus (1 μg/kg) administered at 120 min (GHRP-6-Ghrelin test, Test 3).
Informed consent was obtained from all participants.
Assays
Plasma glucose (Randox, UK, mmol/l) was analyzed by a glucose oxidase method. Plasma GLP-1 levels were determined by elisa (ELISA, Linco Research, USA, pmol/l). The inter- and intra-assay coefficients of variation (CV) were 6.1% and 4.7%, respectively. The normal range was 6-26 pmol/l. INS was determined by radioimmunoassay (RIA, INEP, Zemun, mU/l). The inter- and intra-assay CV were 4.8% and 6.2%, respectively, and the sensitivity of the assay was below 4 mU/ml. The normal levels for plasma INS were 5-25 mU/ml. Serum concentrations of PI were measured by elisa (ELISA, Linco Research, USA, pmol/l), the inter- and intra-asay CV were 4.3% and 6.7%, respectively. The normal levels of PI were 4-20 pmol/l and the sensitivity was 0.5 pmol/l. The C-Peptide (CP) levels were determined by radioimmunoassay (RIA, INEP, Zemun, nmol/l) and the inter- and intra-assay CV were found to be 4.8% and 6.6%, respectively. The sensitivity was 0.08 ng/ml at a 95% confidence limit. The normal levels of CP were 0.4 to 4 nmol/l. Sampling for the various determinations was carried out at time 0 and every 30 min for a period of 240 min.
Statistics
Results are presented as the area under the curve (AUC) and peak values (mean±SE). The incremental glucose insulin, CP, and GLP-1 areas under the curve (AUCs) during the various tests were calculated using the trapezoidal method (with substraction of individual baseline levels) from measurements obtained at the time points indicated above. For the statistical analysis we used the Wilcoxon’s test, ANOVA with Post-Hoc testing, and Spearman correlations. Statistical level of significance was set at p <0.05.
RESULTS
There was no difference in AUC glucose (535.06±14.84 91 mmol · ml-1 · min vs. 526.41±22.91 mmol · ml-1 · min), AUC GLP-1 (2446.0±865.67 pmol · ml-1 · min vs. 2346.87±874.28 pmol · ml-1 · min), AUC INS (588.25±145.46 mU · ml-1 · min vs. 756.25±107.56 mU · ml-1 · min), AUC PI (1206.37±313.65 pmol · ml-1 · min vs. 1276.62±416.15 pmol · ml-1 · min), and AUC CP (158.25±29.75 nmol · ml-1 · min nmol · ml-1 · min vs. 191.87±40.39 nmol · ml-1 · min) during saline infusion and after ghrelin administration (saline-ghrelin test, Test 1).
There was no significant difference in peak levels of INS (8.62±1.28 mU/l vs. 7.23±1.09 mU/l) after ghrelin administration compared to INS levels during saline infusion. There was no difference in peak levels of Glucose (4.40±0.24 mmol/l vs. 4.67±0.19 mmol/l), GLP-1 (21.95±7.54 pmol/l vs. 22.88±8.22 pmol/l), PI (13.55±2.83 pmol/l vs. 15.40±7.03 pmol/l), and CP (1.58±0.23 nmol/l vs. 1.98±0.53 nmol/l) during saline infusion and after ghrelin administration. The levels of glucose, GLP-1, and INS during Test 1 are presented in Figure 1. 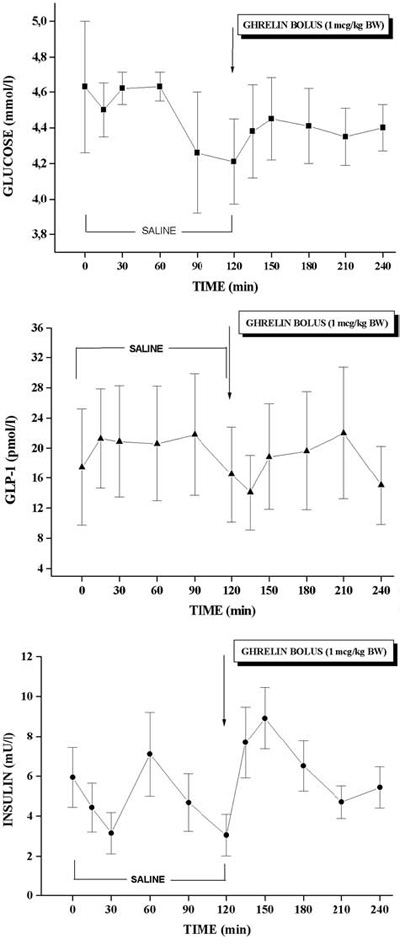
Figure 1. Glucose, GLP-1, and insulin plasma levels during Test 1.
There was a significant difference in AUC Glucose after ghrelin administration in Test 1 and Test 3 (526.41±22.91 mmol · ml-1 · min vs. 566.37±15.64 mmol · ml-1 · min; p<0.05) (Table 1). In contrast, there was no significant difference in AUC Glucose after ghrelin administration compared to AUC Glucose after GHRH (546.87±24.66 mmol · ml-1 · min vs. 559±14.84 mmol · ml-1 · min) and GHRP-6 (566.37±15.64 mmol · ml-1 · min vs. 562 ±16.68 mmol · ml-1 · min ) administration. There was no difference in AUC GLP-1 after ghrelin administration compared to AUC GLP-1 after GHRH (2100.38±965.18 pmol · ml-1 · min vs. 1908.5±873.82 pmol · ml-1 · min) and GHRP-6 (1769.5±784 pmol · ml-1 · min vs. 1963±789.83 pmol · ml-1 · min) administration. There was a significant difference in AUC GLP-1 after ghrelin administration in Test 1 and Test 3 (2346.87±874.28 pmol · ml-1 · min vs. 1769.5±784 pmol · ml-1 · min) (Table 1).
GLP-1 levels during Test 1, Test 2, and Test 3 are presented in Figure 2. 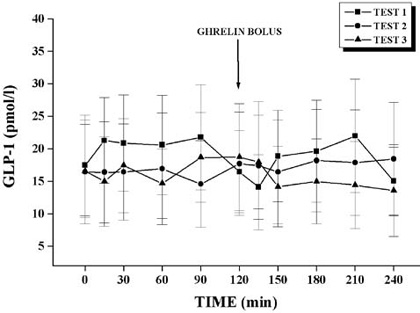
Figure 2. GLP-1 plasma levels in Test 1, Test 2, and Test 3.
There was a significant difference in AUC INS after ghrelin administration (756.25±107.56 mU · ml-1 · min vs. 981. 62±180.32 mU · ml-1 · min ; p< 0.05) in Test 1 and test 3 (Table 1). There was no difference in AUC INS after GHRH (771.75±110.2 mU · ml-1 · min vs. 762.18±85.15 mU · ml-1 · min) and GHRP-6 (981.62±180.32 mU · ml-1 · min vs. 656.93±135.6 mU · ml-1 · min) administration. There was no difference in AUC PI after ghrelin administration compared to AUC PI after GHRH (1248.87±331.04 pmol · ml-1 · min vs. 1834.93±788 pmol · ml-1 · min) and GHRP-6 (1312.35±330 pmol · ml-1 · min vs. 1274.39±382.56 pmol · ml-1 · min) administration. There was no significant difference in AUC PI after ghrelin administration between tests.
There was no difference in AUC CP after ghrelin administration compared to AUC CP after GHRH (192.25±15.73 nmol · ml-1 · min vs. 186.38±25.42 nmol · ml-1 · min) and GHRP-6 (244.50±42.51 nmol · ml-1 · min vs. 195.12±43.13 nmol · ml-1 · min) administration. There was no significant difference in AUC CP after ghrelin administration between tests. The AUCs for glucose, INS, and GLP-1 during all three tests are presented in Figure 3. 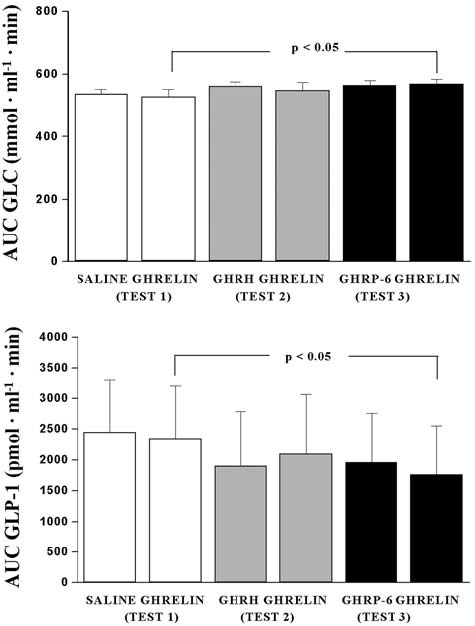
Figure 3. AUCs for Glucose (mmol · ml-1 · min), Insulin (mU · ml-1 · min), and GLP-1 (pmol · ml-1 · min) in Test 1, Test 2, and Test 3.
There were no correlations between peaks and AUCs of glucose, GLP-1, INS, PI and CP after ghrelin administration in any of the three tests.
DISCUSSION
In our study we did not observe any effect of ghrelin administration on hormones of the enteroinsular axis as regards both peak and AUC values. We used a dose of 1 µg/kg BW of ghrelin, i.e. the dose that is routinely used for GH stimulation.17 According to pharmacokinetic data, peak value of ghrelin in the circulation, using this dose, is achieved after 15 minutes (total plasma ghrelin concentration 1058.7 fmol/ml), while the elimination time T1/2 amounts to 27-31 min.18 Based on these data, the observation period of 120 minutes after ghrelin bolus was assumed to be sufficient to observe any stimulatory or inhibitory effect on the investigated parameters in our study.
There was a mild increase in AUC glucose and INS, PI, and CP and a mild reduction in AUC GLP-1 after ghrelin administration, but the differences were not significant. This lack of significance might be explained by the small number of patients involved in the present study. However, there was a significant increase in AUC glucose and INS and a significant decrease in GLP-1 levels when ghrelin was administered after GHRP-6, compared to the ghrelin effect after saline infusion, pointing to an additive effect of GHRP-6 compound on ghrelin action in the entero-pancreatic axis. There is accumulating evidence that ghrelin and GLP-1 are inversely related. In this line, in vitro studies showed suppression of ghrelin secretion after exposure of isolated rat stomach to GLP-1 perfusion.19 These findings may also indicate that, apart from insulin, GLP-1 might be an important factor in postprandial inhibition of ghrelin levels. One possible mode of action could be via somatostatin, considering the fact that GLP-1 stimulates gastric somatostatin secretion.20
In a recent in vitro study, it was shown that insulin and leptin inhibit ghrelin secretion in a dose-dependent manner, while glucagon increases it.16 Analogous results were observed in clinical studies, showing lower ghrelin levels in insulin resistant states and obesity12,21 and demonstrating that insulin infusion further decreases ghrelin levels.8,9 In earlier studies with ghrelin administration, it was shown that ghrelin inhibits insulin secretion,22 although a later study found stimulatory effects of ghrelin on insulin and gastrin secretion.23 In a study conducted in gastrectomized patients, glucose disposal decreased during ghrelin infusion, whereas CP was more suppressed than in the controls and leptin rose significantly, indicating that ghrelin might be involved in the negative control of insulin secretion and glucose utilization in these patients.24 Thus, there is evidence suggesting that ghrelin may modulate circulating glucose levels by releasing growth hormone, increasing insulin resistance, and stimulating gluconeogenesis and could be strongly involved in energy homeostasis.25,26
In conclusion, there was no evidence of any direct effect of ghrelin on the enteroinsular axis in this study. Bearing in mind the limited number of studies evaluating this problem and the fact that changes, albeit small, in glucose, INS, and GLP-1 levels were observed in this study after ghrelin was combined with GHRH-6 administration, further investigations should be conducted focusing on ghrelin-pancreas/gut hormones interplay.
REFERENCES
1. Date Y, Kojima M, Hosoda H, et al, 2000 Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141: 4255-4261.
2. Petersen S, 2002 Growth hormone secretagogues and ghrelin: an update on physiology and clinical relevance. Hormone Research 58: Suppl 3: 56-61.
3. Ghigo E, Broglio F, Arvat E, et al, 2005 Ghrelin: more than a natural GH secretagogue and/or an orexigenic factor. Clin Endocrinol 62: 1-17.
4. Kojima M, Hosoda H, Date Y, et al, 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656-660.
5. Ariyasu H, Takaya K, Tagami T, et al, 2001 Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86: 4753-4758.
6. Tschop M, Smiley DL, Helman ML, 2000 Ghrelin induces adiposity in rodents. Nature 407: 908-912.
7. Nakazato M, Murakami N, Date Y, et al, 2001 A role for ghrelin in the central regulation of feeding. Nature 409: 194-198.
8. Broglio F, Benso A, Castiglioni C, et al, 2003 The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab 88: 1537-1542.
9. Cummings DE, Weigle DS, Frayo S, et al, 2002 Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623-1630.
10. Flanagan DE, Evans ML, Monsod TP, et al, 2001 The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab 284: E313-16.
11. Micic D, Sumarac-Dumanovic M, Cvijovic G, et al, 2004 Ghrelin levels during euglycemic hyperinsulinemic clamp in women with polycystic ovary syndrome. International Proceedings: 12th International Congress of Endocrinology, MEDIMOND, Bologna, Italy, 2004: 879-882.
12. Broglio F, Gottero C, Prodam F, et al, 2004 Ghrelin secretion is inhibited by glucose load and insulin-induced hypoglycaemia but unaffected by glucagon and arginine in humans. Clin Endocrinol 61: 503-509.
13. Broglio F, Gottero C, Prodam F, et al, 2004 Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab 89: 3062-3065.
14. Eissele R, Koop H, Arnold R, 1990 Effect of glucagon-like peptide-1 on gastric somatostatin and gastric secretion in the rat. Scand J Gastroenterol 25: 449-454.
15. Flint A, Raben A, Astrup A, et al, 1998 Glucagon-like peptide-1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101: 515-520.
16. Djurhuus CB, Hansen TK, Gravholt C, et al, 2002 Circulating levels of ghrelin and GLP-1 are inversely related during glucose ingestion. Horm Metab Res 34: 411-413.
17. Larsen PJ, Tang-Christensen M, Holst JJ, et al, 1997 Distribution of glucagon-like pepetide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257-270.
18. Kamegai J, Tamura H, Shimizu T, et al, 2004 Effects of insulin, leptin, and glucagon on ghrelin secretion from isolated perfused rat stomach. Regul Pept 119: 77-81.
19. Lippl F, Kircher F, Erdmann J, et al, 2004 Effect of GIP, GLP-1, insulin and gastrin on ghrelin release in the isolated rat stomach. Regul Pept 119: 93-98.
20. Egido EM, Silvestre RA, Hernandez R, Marco J, 2004 Exendin-4 dose dependently stimulates somatostatin and insulin secretion in perfused rat pancreas. Horm Metab Res 36: 595-600.
21. McLaughlin T, Abbasi F, Lamendola C, et al, 2004 Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab 89: 1630-1635.
22. Broglio F, Arvat E, Benso A, et al, 2001 Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 86: 5083-5086.
23. Lee HM, Wang G, Englander EW, et al, 2002 Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 143: 185-190.
24. Damjanovic SS, Lalic NM, Pesko PM, et al, 2006 Acute effects of ghrelin on insulin secretion and glucose disposal rate in gastrectomized patients. J Clin Endocrinol Metab 91: 2574-2581.
25. Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB, 2004 Ghrelin – a hormone with multiple functions. Frontiers in Neuroendocrinol 25: 27-68.
26. Broglio F, Prodam F, Riganti F, et al, 2006 Ghrelin: from somatotrope secretion to new perspectives in the regulation of peripheral metabolic functions. Front Horm Res 35: 102-114.
Address for correspondence:
Micic Dragan, Institute of Endocrinology, Diabetes and
Diseases of Metabolism, Dr Subotica 13, 11000 Belgrade,
Serbia, Tel.: + 381-11-656527, Fax: + 381-11-3065081,
e-mail: micicd@eunet.yu
Received 07-05-07, Revised 10-08-07, Accepted 10-09-07