1National Research Center for Endocrinology of the Russian Academy of Medical Science, Moscow, 2Department of Endocrinology of Moscow Medical Academy, Moscow, 3Moscow Endocrinology Unit, Moscow, Russia
OBJECTIVE: To evaluate the effects of subclinical hyperthyroidism of variable etiology on bone mineral density (BMD) and bone metabolism in postmenopausal women. DESIGN: T he study included data of 88 postmenopausal women classified into four groups depending on the etiology of subclinical hyperthyroidism: (1) 20 with toxic multinodular goiter without history of clinical hyperthyroidism; (2) 25 on levothyroxine suppressive therapy after thyroidectomy due to differentiated thyroid cancer; (3) 21 with Graves’ disease (GD) receiving antithyroid drugs; (4) 22 healthy women matched for age and duration of menopause. In all subjects biochemical markers of bone turnover and B MD were determined. RESULTS: B iochemical markers of bone turnover were significantly higher (p-value =0.001) in all patients with subclinical hyperthyroidism compared to the control group (group 4). T he women of group 1 had significantly lower B MD at all regions of the skeleton, whereas the women of group 3 had significantly lower B MD at T otal Hip (p-value = 0.013) and R adius T otal (p-value = 0.0003) compared to group 4. No significant differences in B MD between groups 2 and 4 were detected. CONCLUSION: The etiology of subclinical hyperthyroidism influences B MD in postmenopausal women. Endogenous subclinical hyperthyroidism might be considered as an additional risk factor for osteoporosis in postmenopausal women, especially for cortical bone, whereas exogenous subclinical hyperthyroidism has no effect on BMD.
Bone mineral density, Bones, C-terminal telopeptide of type I collagen, Osteocalcin, Osteoporosis, Postmenopausal women, Subclinical hyperthyroidism, Thyrotropin
INTRODUCTION
The clinical consequences of overt hyperthyroidism on the skeleton were first suggested over 100 years ago (1891) when Von Recklinghausen reportedapatientwithhyperthyroidismandmultiple fractures.1 However, today with earlier recognition of thyroid disease and its effective treatment, severe bone loss is rarely seen.1 In recent years, with the development of sensitive thyrotropin (TSH) assays, it has been suggested that even subclinical hyperthyroidism may have effects on fracture risk.2,3 Extra-thyroidal thyrotropin receptors (TSHR) have been demonstrated in several tissues and cells, including human and rat osteosarcoma cell lines and primary cultures of human osteoblast-like (hOB) cells, though Tsai et al concluded that TSH effects had no physiological relevant effects on hOB cells.4-8
Subclinical hyperthyroidism, defined as free triiodothyronine (FT3) and free thyroxine (FT4) concentrations within the reference range and serum TSH level below the reference range,9 seems to be an appropriate model to examine the direct effect of TSH on bone in clinical practice. Several recent studies, designed with the aim of finding new risk factors for osteoporosis, have shown that post menopausal women with low TSH level (the etiology of subclinical hyperthyroidism was not specified) have lower bone mineral density(BMD)and higher fractureriskcom-pared to women with normal TSH.10-12 Thus, a secondary analysis of data collected as part of the Fracture Intervention Trial (FIT), which included 15,316 postmenopausal women, concluded that women with TSH <0.1mlU/l as well as TSH <0.5mlU/l had significantly lower BMD at the femoral neck and in total body as compared to women with normal TSH.10 Furthermore, a cross-sectional, hospital-based survey, in which 959 healthy postmenopausal women were enrolled, showed that women even with low normal TSH levels (0.5-1.1mU/l) had significantly lower BMD at the lumbar spine and femoral neck than those with high normal TSH levels (2.8-5.0).11 Additionally, a cross-sectional study in Seoul, Korea, which enrolled 413 postmenopausal women, found that femoral neck BMD was significantly reduced both in the subclinical hyperthyroid group and in the subclinical hypothyroid group as compared with the euthyroid group.12 However, a prospective cohort study of 458 women over age 65 participating in the multicenter Study of Osteoporotic Fractures, found no consistent evidence that low TSH was associated with low BMD or accelerated bone loss in older ambulatory women.13 According to a summary of the evidence for the U.S. Preventive Services Task Force and several other reviews, the issue as to whether subclinical hyperthyroidism has a clinically significant effect on BMD and fracture risk remains controversial.14-16
The aim of the present study was to determine BMD and bone metabolism in postmenopausal women with subclinical hyperthyroidism of various etiology.
SUBJECTS AND METHODOLOGY
Eighty-eight postmenopausal women (at least 5 years after menopause) were classified into four groups: (1) 20 women with toxic multinodular goiter (TMG) without history of clinical hyperthyroidism; (2) 25 women on Levothyroxine (LT4) suppressive therapy 125 (125-150) μg/day (Median (Me) Lower-upper quartiles (Q25-Q75)) during a mean period of 3 (1.5-6) years post thyroidectomy for differentiated thyroid cancer; (3) 21 women with Graves’ disease (GD) and overt hyperthyroidism in the anamnesis, receiving antithyroid agents during a mean period of 3 (2-7) years, having normal FT3 and FT4 levels for at least 6 months prior to the study as well as sus-tained serum TSH suppression long after antithyroid treatment; and (4) 22 healthy women without thyroid dysfunction at present or by history and matched for age and duration of menopause (controls). All women were studied as out patients during a one-year period. The presence of low TSH and normal FT3, FT4 levels was established by two measurements.
All participants were interviewed and examined during the baseline visit. At that visit demographic and somatometric data as well as detailed data about physician-diagnosed medical conditions and past and current medication use were collected. All women were also asked about the presence of risk factors for osteoporosis such as prior fragility fractures, family history of osteoporosis, high caffeine intake, low calcium intake, smoking, physical activity in youth and at present, the number of full-term pregnancies and the duration of breast feeding. Biochemical parameters: calcium (Ca), phosphorous (P), creatinine (Cr), alkaline phosphatase (ALP), cholesterol, low density lipoprotein (LDL), high density lipoprotein (HDL), triglycerides (TG), cholesterol/HDL ratio in fasting serum as well as calcium/creatinine ratio in fasting urine specimen (U-Ca/U-Cre) were measured using standard laboratory methods on the biochemical analyzer Hitachi 912. FT3, FT4 and TSH were measured using commercially available kits (Vitros Eci, Ortho-Clinical Diagnostics Amersham, UK). TSH-receptor antibodies were detected with the h-TBII assay (“TRAK-Human DYNO-test” (BRAMS AG, Germany) in groups 1 and 3 so as to differentiate GD and TMG. Biochemical markers of bone metabolism: serum osteocalcin (OC) and C-terminal telopeptide of type I collagen (β-CTx) as well as parathyroid hormone (PTH) levels were measured using the electrochemiluminescence immunoas-say “ECLIA” on the Roche Elecsys 1010/2010 and Modular Analytics E170.
BMD was measured by dual energy X-ray absorptiometry (DXA)(Prodigy,Lunar)atthelumbarspine (L1-L4), femoral neck, total hip and radius total.
Statistical analysis: STATISTICA 6.0 Stat Soft was used in the analysis. The data with normal dis-tributions are expressed as means with 95% confidence limits of means, otherwise as medians with interquartile ranges. The level of significance was set at 5%. Comparisons of the variables among different groups were made with one-way ANOVA, and the multiple comparison tests were performed with post-hoc analyses (the Fisher LSD test) for data with normal distribution, otherwise with the Kruscal-Wal-lis ANOVA test, and the Mann-Whitney U-test with the Bonferroni correction. The Spearman test was used for the correlation analysis.
RESULTS
There were no differences in the age, menopause duration, height, weight and body mass index (BMI) as well as prior fragility fracture, family history of osteoporosis, high caffeine intake, low calcium intake, smoking, physical activity in youth and at present, the number of full-term pregnancies and the duration of breast feeding among the four groups of studied women. The characteristics of the participants at baseline are presented in Table 1 . The FT3 and FT4 levels were within the reference range. There were no differences in FT3 (p-value=0.267) and PTH (p-value=0.258) levels among the groups. However, FT4 levels were significantly higher in group 2 versus group 4 (p-value <0.001), while FT4 levels in groups 1 and 3 were not different and were comparable to group 4 (p-value=0.718 and 0.420, respectively). No differences in TSH values were found among groups 1, 2 and 3 (p-value=0.614). TSH levels in groups 1, 2 and 3 were significantly lower than those in group 4 (Table 2).
Amongthefourgroups,therewerenodifferences in biochemical parameters except for ALP levels, which were significantly higher (p-value=0.019) in patients with GD compared to the control group (Table 2).
Biochemical markers of bone turnover were sig-nificantly higher in all three groups of patients with subclinical hyperthyroidism compared to group 4 (Table 2). OC level was 56.3% higher in group 1 (p-value <0.001), 28% higher in group 2 (p-value= 0.005) and 50.2% higher in group 3 (p-value <0.001) compared to the control group. CTx level was 84 % higher (p-value=0.002) in group 1, 36.2% higher (p-value=0,050) in group 2 and 85.5% higher (p-value <0.001) in group 3 compared to the control group.
For all patients enrolled in this study TSH exhibited a significant negative correlation with OC (r=-0.38; p-value <0.001) and β-CTx (r=-0.36; p-value<0.001) levels (Figure 1). A significant positive correlation was detected between FT3 values and OC (r=0.28; p-value=0.007) and β-CTx (r=0.24; p-value=0.023) values (Figure 2) as well as between PTH and OC (r=0.28; p-value=0.009) and .-CTx (r=0.23; p-value=0.034) values. 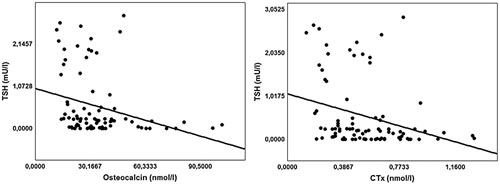
Figure 1. Negative correlations of serum TSH with Oscteocalcin and CTx levels for all patients enrolled in the study; Osteocalcin r(Spearmen)=-0.38; p-value <0.001, C-terminal telopeptide of type I collagen (CTx) r(Spearmen)=-0.36; p-value<0.001.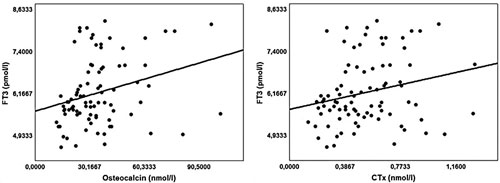
Figure 2. Positive correlations of serum FT3 with Osteocalcin and CTx values for all patients enrolled in the study. Osteocalcin r(Spearmen) = 0.28; p-value=0.007, C-terminal telopeptide of type I collagen (CTx) r(Spearmen)=0.24; p-value=0.023.
BMD values differed significantly at all parts of the skeleton among the four groups. Spine BMD (L1-L4) was 7.6% lower (p-value= 0.044) (Figure 3) and Neck BMD was 8.6% lower (p-value=0.034) in group 1 versus the control group (Figure 4). Total Hip BMD was 10.8% lower (p-value=0.01) in group 1 and 10.3% lower (p-value=0.013) in group 3 versus group 4 (Figure 4). Radius Total BMD was 11.1% lower (p-value= 0.006) in group 1 and 17.3% lower (p-value <0.001) in group 3 compared to the control group (Figure 5). The Post-hoc analysis also showed that BMD was significantly lower in group 1 (at L1L4 (p-value=0.023); at Neck (p-value=0.005); at Total Hip (p-value=0.006); at Radius Total (p-value <0.001)) as well as in group 3 (at L1-L4 (p-value= 0.033); at Neck (p-value=0.042); at Total Hip (p-value=0.008); at Radius Total (p-value <0.001) versus group 2 (Figures 3, 4, 5). There were no sig-nificant differences in BMD between groups 1 and 3 nor between groups 2 and 4. 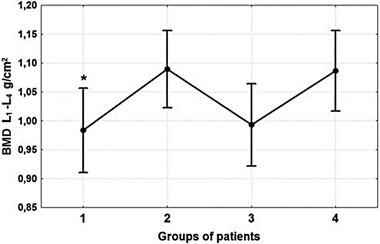
Figure 3. Comparative analysis of L1-L4 BMD. The one-way ANOVA test did not show significant differences among the four groups (p-value=0.053). However, Post-hoc analysis showed that *Spine BMD (L1-L4) was 7.6% lower (p-value= 0.044) in group 1 compared to the control group. Spine BMD (Post-hoc analysis) was significantly lower in group 1 (pvalue= 0.023) and group 3 (p-value=0.033) compared to group 2. No significant differences were detected between groups 1 and 3, and between groups 2 and 4.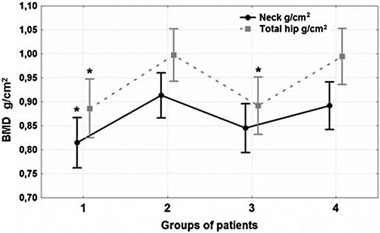
Figure 4. Comparative analysis of Neck and Total Hip BMD. Significant differences among the four groups (one-way ANOVA) both at Neck (p-value=0.028) and at Total Hip (p-value= 0.006) were detected. Neck BMD was 8.6% lower (p-value =0.034) in group 1, and Total Hip BMD was 10.8% lower (pvalue= 0.017) in group 1 and 10.3% lower (p=0.013) group 3 versus group 4. It was also found (Post-hoc analysis) that Neck BMD was significantly lower in group 1 (p-value=0.005) and group 3 (p-value=0.042) versus group 2. BMD at Total Hip was also significantly lower in group 1 (p-value=0.006) and group 3 (p-value=0.008) in comparison with group 2. There were no significant differences in BMD among groups 1 and 3, and groups 2 and 4.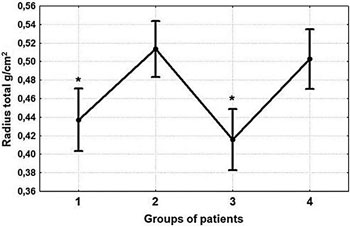
Figure 5. Comparative analysis of Radius Total BMD. Significant differences among the four groups (one-way ANOVA) (pvalue= 0.001) were detected. *Radius Total BMD was 11.1% lower (p-value=0.006) in group 1 and 17.3% lower (p-value= <0.001) in group 3 compared to the control group. Post-hoc analysis showed that BMD at Radius Total was lower in group 1 (p-value <0.001) as well as in group 3 (p-value=<0.001) versus group 2. Groups 1 and 3 did not significantly differ, nor did groups 2 and 4.
DISCUSSION
Overt hyperthyroidism is associated with increased bone resorption, low bone mineral density and increased number of fractures in postmeno-pausal women.17,18 Whether endogenous or exogenous subclinical hyperthyroidism decreases BMD or increases fracture rate remains controversial.14,16,19,20 Studies examining the effects of exogenous subclinical hyperthyroidism on bone density have been inconsistent. The results of two meta-analyses (1994 and 1996) showed significant bone loss in postmenopausal women on LT4 suppressive therapy versus the control group.21,22 However, two recent systematic reviews of the literature showed that BMD changes in postmenopausal women on LT4 suppressive therapy remain unclear and require further evaluation.19,20
Results of bone density studies in subjects with endogenous subclinical hyperthyroidism are scarce. One case-control study showed a significant increase in bone turnover markers and a decrease in bone mass in postmenopausal women with endogenous subclinical hyperthyroidism due to TMG,23 whereas such an outcome was only suggestive in another paper which included both postmenopausal and premenopausal women.24
Two recent studies examined bone turnover25 and BMD26 in premenopausal women affected by GD and who had subclinical hyperthyroidism at the time of evaluation. The markers of bone metabolism were significantly higher in the group with suppressed TSH compared with the group with normal TSH,25 where as no differences in BMD were detected among patients with subclinical hyperthyroidism and the control group.26
Bone turnover and BMD were determined in 49 male patients with treated thyroid cancer on LT4 suppressive therapy (17 patients) and in newly diagnosed GD (32 patients).27 Bone markers were significantly higher in patients with GD, whereas no significant differences in BMD were found among the groups.27
The primary aim of the present study was to compare the bone metabolism and BMD in postmenopausal women in relation to the etiology of subclinical hyperthyroidism. The implemented design targeted the differences not only between subjects with subclinical hyperthyroidism and the control group, but also among the groups of patients with both exogenous and autoimmune and non autoimmune endogenous subclinical hyperthyroidism.
The markers of bone metabolism levels were significantly higher in all patients with subclinical hyperthyroidism versus healthy postmenopausal women. Significant correlations of markers of bone turnover with TSH, FT3, and PTH, but not FT4, levels were also detected.
Positive correlations of PTH and FT3 levels (which were within the reference range for all enrolled women) with markers of bone metabolism reflected their normal physiological influence on bone.28-32 At physiological levels, T4 is inactive because it possesses 100-fold lower affinity than T3 for binding to the thyroid hormone receptor (TR).31 It was thus expected that no correlation of FT4 with markers of bone metabolism was found.
The negative correlation of TSH and the mark-ers of bone turnover might be considered as indirect evidence of an influence of TSH on bone along with FT3. Recently published data demonstrated a critical role for TSH in skeletal remodeling, which is independent of its effects on circulating thyroid hormones.4,7 Using mice in which the TSHR gene had been knockout, Abe et al found that BMD, in the presence of normal levels of thyroid hormone, depends on an intact response to TSH.4 According to these findings, TSH might be defined as a negative regulator of skeletal remodeling, which exerts influence through its receptor on both osteoblast and osteoclast precursors.4,7,33 Besides, a recent study in elderly humans enrolled in the Rotterdam Study showed that the TSHR ASP727GLU allele, which results in an increased cAMP response of the recep-tor to TSH, is associated with higher BMD and bone mineral content (BMC).34
These results possibly offer an explanation for the increase in the markers of bone metabolism levels in patients with low TSH.
However, in our study only women with endogenous subclinical hyperthyroidism had significantly lower BMD versus the control group, whereas there were no significant differences in BMD between patients with exogenous subclinical hyperthyroidism and the control group. Therefore, it is likely that not only low TSH level but also the etiology of subclinical hyperthyroidism affect BMD.
It is evident, however, that the women with GD had high thyroid hormone values for a certain period in the past, whereas the patients from groups 1 and 2 did not report overt hyperthyroidism at any time. One may expect that this period of hyperthyroidism in the GD group might have adversely affected bone remodeling. Reconstruction of the bone remodeling sequence in uncontrolled hyperthyroid patients indicates a net loss of about 10-15% of mineralized bone per bone remodeling cycle.35 Completion of a full remodeling cycle normally lasts about 6 months.32 Usually, about 90% of “bone remodeling units” on bone surfaces is dormant, whereas the rate of turnover of the whole skeleton is about 10% per year.36 Consequently, thyroid hormone levels need to remain high for an average of 12 months to cause significant bone loss (2% per year). Therefore, severe bone loss due to overt hyperthyroidism is now uncommonly seen. Patients included in group 3 had normal FT3 and FT4 levels and were under the observation of endocrinologists for a mean period of 3 years (2-7 years) prior to entering the study. Thus, we considered them as patients with endogenous autoimmune subclinical hyperthyroidism.
Indeed, women with endogenous subclinical hyperthyroidism (groups 1 and 3) had a similar decrease in BMD,predominantly indistal and proximal cortical bone, though group 1 did not have a history of overt hyperthyroidism in the past. Women with exogenous subclinical hyperthyroidism did not differ from the control group. To explain this finding, other TSH effects should be considered. It has been found that TSH exerts influence not only on the bone precursors but on the mature bone cells as well. In a recent study, iodothyronine deiodinase activity, which is essential for conversion of FT4 to FT3 and is regulated by a TSH receptor-cAMP mediated mechanism,37 was detected in human osteoblast-like osteosarcoma (SaOS-2) cells as well as in normal human osteoblast (NHOst) cells.6 All the characteristics of the deiodinating activity were compatible with Type 2 iodothyronine deiodinase (D2).6 D2 activity was stimulated by TSH in both cells’ cultures.6 Hence, this study suggested that TSH receptors in bone cells are responsible for the local T3 production by D2. The daily secretion of the normal thyroid gland is about 100 nmol of T4, 5 nmol of T3 and less than 5 nmol of reverse T3.32 Most of the plasma pool of T3 is derived from peripheral metabolism of T4.31 Thus, the low TSH level may lead to the decrease of local T3 production in all patients with subclinical hyperthyroidism, but relatively higher T3 production might be expected in patients with endogenous subclinical hyperthyroidism, which is not observed in patients after total thyroidectomy. Therefore, inspite of significant lyhigher FT4 level in patients with exogenous subclinical hyperthyroidism, no differences in BMD were found compared with the control group. However, it is necessary to emphasize that FT3 levels were in the reference range and were not significantly different among the various groups of patients.
Due to lack of clinical symptoms, it is impossible to justify the exact duration of TSH suppression in group 1.
In conclusion, the etiology of subclinical hyperthyroidism possibly affects BMD in postmenopausal women. Postmenopausal women with endogenous subclinical hyperthyroidism have significantly lower BMD, mainly at the distal and proximal cortical bone,versus healthy postmenopausal women. Therefore, endogenous subclinical hyperthyroidism (both of autoimmune and non autoimmune etiology) might be considered as an additional risk factor for oseoporosis in postmenopausal women. On the other hand, exogenous subclinical hyperthyroidism has no effect on BMD. Postmenopausal women with subclinical hyperthyroidism have a higher bone turnover rate in comparison with the control group, though they do not differ in biochemical parameters of calcium and phosphorous metabolism.
REFERENCES
1. Murphy E, Williams GR, 2004 The thyroid and the skeleton. J Clin Endocrinol 61: 285-298.
2. Klee GG, Hay ID, 1988 Sensitive thyrotropin assays: analytic and clinical performance criteria. J Mayo Clin Proc 63: 1123-1132.
3. Bauer DC, Nevitt MC, Ettinger B, Stone KL, 2001 Risk for fracture in women with low serum levels of thyroid-stimulating hormone. J Ann Intern Med 134: 561-568.
4. Abe E, Marians RC, Yu W, et al, 2003 TSH is a negative regulator of skeletal remodeling. Cell 115: 151-162.
5. Inoue M, Tawata M, Yokomori N, Endo T, Onaya T, 1998 Expression of thyrotropin receptor on clonal osteoblast-like rat osteosarcoma cells. J Thyroid 8: 1059-1064.
6. Morimura T, Tsunekawa K, Kasahara T, et al, 2005 Expression of type 2 iodothyronine deiodinase in human osteoblast is stimulated by thyrotropin. J Endocrinology 146: 2077-2084.
7. Norvack DV, 2003 TSH the bone suppressing hormone. J Cell 115: 129-130.
8. Tsai JA, Janson A, Bucht E, et al, 2004 Weak evidence of thyrotropin receptors in primary cultures of human osteoblast-like cells. J Calcif Tissue Int 74: 486-491.
9. Surks MI, Ortiz E, Daniels GH, et al, 2004 Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 291: 228-238.
10. Jamal SA, Leiter RE, Bayoumi AM, Bauer DC, Cummings SR, 2005 Clinical utility of laboratory testing in women with osteoporosis. J. Osteoporos Int 16: 534-540.
11. Kim DJ, Khang YH, Koh JM, Shong YK, Kim CS, 2006 Low normal TSH levels are associated with low bone mineral density in healthy postmenopausal women. J Clin Endocrinol (Oxf) 64: 86-90.
12. Lee WY, Oh KW, Rhee EJ, et al, 2006 Relationship between subclinical thyroid dysfunction and femoral neck bone mineral density in women. J Arch Med Res 37: 511-516.
13. Bauer DC, Nevitt MC, Ettinger B, Stone K, 1997 Low thyrotropin levels are not associated with bone loss in older women: a prospective study. J Clin Endocrinol Metab 82: 2931-2936.
14. Biondi B, Palmieri EA, Klain M, Schlumberger M, Filetti S Lombardi G, 2005 Subclinical hyperthyroidism: clinical features and treatment options. Eur J Endocrinol 152: 1-9.
15. Helfand M, 2004 Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. J Ann Intern Med 140: 128-141.
16. Papi G, Pearce EN, Braverman LE, Betterle C, Roti E, 2005 A clinical and therapeutic approach to thyrotoxicosis with thyroid-stimulating hormone suppression only. Am J Med 118: 349-361.
17. Vestergaard P, Mosekilde L, 2003 Hyperthyroidism, Bone Mineral, and Fracture Risk – a meta-analysis. Thyroid 13: 585-593.
18. Akalin A, Colakt O, Alatast O, Efe B, 2002 Bone remodeling markers and serum cytokines in patients with hyperthyroidism. J Clin Endocrinology 57: 125-129.
19. Quan ML, Pasieka JL, Rorstad O, 2002 Bone mineral density in well-differentiated thyroid cancer patients treated with suppressive thyroxine: a systematic overview of the literature J Surg Oncol 79: 62-70.
20. Schneider R, Reiners C, 2003 The effect of levothyroxine therapy on bone mineral density: a systematic review of the literature. Exp Clin Endocrinol Diabetes 111: 455-470.
21. Faber J, Galloe AM, 1994 Changes in bone mass during prolonged subclinical hyperthyroidism due to L-thyroxine treatment: a meta-analysis. Eur J Endocrinol 130: 350-356.
22. Uzzan B, Campos J, Cucherat M, Nony P, Boissel JP, 1996 Effects on bone mass of long term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab 81: 4278-4289.
23. Tauchmanova L, Nuzzo V, Del Puente, et al, 2004 Reduced bone mass detected by bone quantitative ultrasonometry and DEXA in pre-and postmenopausal women with endogenous subclinical hyperthyroidism, Maturitas, 48: 299-306.
24. Foldes J, Tarjan G, Szathmari M, Varga F, Krasznai I, Horvath C, 1993, Bone mineral density in patients with endogenous subclinical hyperthyroidism: is this thyroid status a risk factor for osteoporosis? J Clin Endocrinol 39: 521-527.
25. Kumeda Y, Inaba M, Tahara H, et al, 2000 Persistent increase in bone turnover in Graves’ patients with sub-clinical hyperthyroidism. J Clin Endocrinol Metab 85: 4157-4161.
26. Ugur-Altun B, Altun A, Arikan E, Guldiken S, Tugrul A, 2003 Relationships existing between the serum cyto-kine levels and bone mineral density in women in the premenopausal period affected by Graves’ disease with subclinical hyperthyroidism. Endocr Res 29: 389-398.
27. Jodar E, Martinez-Diaz-Guerra G, Hawkins AF, 2001 Bone mineral density in male patients with L-Thyroxine suppressive therapy and Graves disease. Calcif Tissue Int 69: 84-87.
28. Pereira RC, Jorgetti V, Canalis E, 1999 Triiodothyronine induces collagenase-3 and gelatinase B expression in murine osteoblasts. Am J Physiol 277: E496-E504.
29. Salto C, Kindblom JM, Johansson C, et al, 2001 Ablation of TRα and a concomitant overexpression of α1 yields a mixed hypo and hyperthyroid phenotype in mice. J Mol Endocrinol 15: 2115-2128.
30. Siddiqi A, Burrin JM, Wood DF, Monson JP, 1998 Tri-iodothyronin regulates the production of interleukin-6 and interleukin-8 in human bone marrow stromal and osteoblast-like cells. J Endocrinology 157: 453-461.
31. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR, 2002 Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronin selenodeiodinases. J Endocr Rev 23: 38-89.
32. Greenspan FS, Gardner DG 2004 Basic and clinical endocrinology. Seventh Edition. Lange Medical Books, McGraw-Hill; pp, 216-232.
33. Sun L, Davies TF, Blair HC, Abe E, Zaidi M, 2006 TSH and bone loss. Ann NY Acad Sci 1068: 309-318.
34. Deure VWM, Uitterlinden AG, Pols HAP, Peeters RP, Visser TJ, 2005 The TSH receptor ASP727GLU polymorphism is associated with higher bone mineral density and bone mineral content. J Thyroid 15: s20-s21.
35. Bassett JH, Williams GR, 2003 The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab 14: 356-364.
36. Manolagas SC, 2000 Birth and death of bone cells: basic regulatory mechanisms and implications for the patho-genesis and treatment of osteoporosis. Endocr Rev 21: 115-137.
37. Muracami M, Araki O, Hosoi Y, et al, 2001 Expression and regulation of type II iodothyronine deiodinase in human thyroid gland. J Endocrinology 142: 2961-2967.
Address for correspondence:
Zhanna Belaya, National Research Center for Endocrinology
RAMS, Dmitriya Uljanova, 11, Moscow, Russia 117036,
Tel.: 7(495)1244302, Fax: 7(495)5000092,
e-mail: janne-be@mtu-net.ru; zhannabelaya@yahoo.co.uk
Received 07-11-06, Revised 11-12-06, Accepted 15-12-06